Giant cell arteritis (GCA) is a relatively common rheumatologic disease with potentially devastating complications. It was first described in the early 1890s; however, more than a hundred years later, this large- and medium-sized vessel vasculitis continues to pose multiple challenges for rheumatologists and patients in terms of diagnosis and therapy, as well as the management and prevention of treatment complications.
GCA is the most common form of primary vasculitis among adults in Western countries, with a prevalence ranging from 24 to 280 cases per 100,000 individuals older than 50. The incidence of GCA increases progressively with age and peaks during the seventh decade of life. The disease has a particular predilection for elderly populations of northern European ancestry and is approximately twice as common in women than in men.1
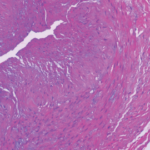
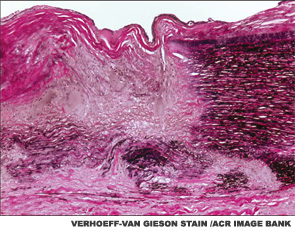
The main histopathologic feature of GCA comprises a chronic granulomatous inflammatory process that involves the aorta and its main branches, with a tendency to affect the extracranial carotid arteries and the ophthalmic circulation (see Figure 1). Its clinical manifestations consist of constitutional symptoms (e.g., asthenia, fever), headaches, jaw claudication, shoulder and hip girdle pain and stiffness (i.e., polymyalgia rheumatica) and elevated acute-phase reactants.2
The most feared consequence of GCA pertains to vision loss, occurring in 15–20% of cases. Blindness, which tends to occur before the disease is even suspected, usually occurs as the result of anterior ischemic optic neuropathy (AION) due to the occlusion of the long posterior ciliary arteries that perfuse the head of the optic nerve.
Other possible complications include scalp and tongue necrosis, aortic aneurysm, aortic dissection, limb claudication from large-artery stenosis (particularly the subclavian and axillary arteries), stroke, mesenteric ischemia, myocardial infarction and venous thromboembolism.2
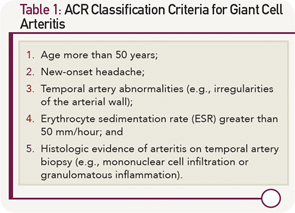
Classification Criteria
In 1990, the ACR classification criteria were established for the purpose of differentiating GCA from other vasculitides.3 The five components of the ACR classification criteria for GCA are shown in Table 1 (p. 22). Fulfillment of at least three of the five ACR criteria is associated with a 90% sensitivity and a 90% specificity for GCA when evaluating patients known to have one form of systemic vasculitis or another. The ACR classification criteria were not designed for use as diagnostic criteria, but rather for the purpose of ensuring greater uniformity of patient populations in studies of vasculitis.
Rendering the diagnosis of GCA is often considerably more challenging than referring to the features of the ACR classification criteria, however. The evaluation of cranial symptoms—common complaints in clinic populations—requires substantial experience and judgment.
Moreover, the detection of temporal artery abnormalities by physical examination is highly dependent on the skill of the examiner, and up to one-third of patients with biopsy-proven GCA in the temporal arteries have normal temporal artery findings on physical examination.
In addition, up to 10% of the GCA patients have either normal inflammatory markers or falsely negative temporal artery biopsies, even before the initiation of corticosteroid (CS) therapy.4,5
Non-Invasive Vascular Imaging
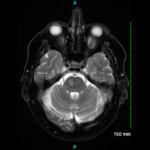
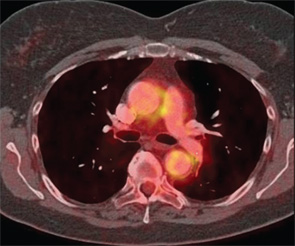
Since the development of the ACR classification criteria for GCA, cross-sectional imaging has assumed an increasing role in the evaluation of patients with large-vessel vasculitis. Current radiologic modalities, which include magnetic resonance imaging (MRI), magnetic resonance angiography (MRA), computed tomography angiography (CTA), positron emission tomography (PET) and combinations of those (e.g., PET/CT, PET/CTA or PET/MRI/MRA) (see Figure 2), can detect mural and luminal vascular abnormalities indicative of active or previous “vasculitic damage” to elastic and muscular arteries. These abnormalities include arterial wall thickening, edema or contrast/radioactive isotope uptake, as well as segments of narrowing, occlusion or aneurysmal dilatation. For example, vascular imaging may suggest GCA in elderly patients presenting with limb claudication and increased inflammatory markers in the absence of cranial symptoms, polymyalgia rheumatica or temporal artery abnormalities. In addition, vascular radiology is useful in individuals with known GCA who develop thoracic aortic aneurysms and, therefore, need longitudinal image surveillance to plan the timing of surgery to prevent aortic rupture.
Depending on the clinical setting (e.g., new-onset vs. long-standing disease) and the technology used, between 10% and 90% of all GCA patients have radiographic evidence of disease in the aorta and its primary branches. In early stages of the disorder, arterial wall thickening and/or mural contrast enhancement is demonstrated in up to 70% of subjects by CTA.6
Acute arteritic lesions are also seen by MRA/MRI in more than 90% of cases presenting with fever of unknown origin.7 On follow-up, chronic stenosis and/or dilatations of the thoracic aorta, subclavian and axillary arteries are frequently detected by MRA.8
On the other hand, nearly 80% of GCA patients have long segments of increased linear 18F-fluorodeoxyglucose (18F-FDG) uptake by PET in at least one vascular territory at diagnosis.9 The subclavian arteries are involved in three out of four cases and the aorta in almost half of the individuals.
Other vessels that may display increased metabolic activity include the brachiocephalic trunk, axillary arteries, carotid arteries, iliac arteries and femoral arteries. A small meta-analysis has suggested that PET has a positive predictive value of 0.85 (95% CI 0.62, 0.95) and a negative predictive value of 0.88 (95% CI 0.72, 0.95) for the diagnosis of large-vessel vasculitis.10
Although the utility of cross-sectional arterial imaging for the diagnosis of GCA is high, a limited amount of research exists regarding the usefulness of vascular radiology for the purpose of evaluating response to treatment, disease activity, risk of disease flare or likelihood of long-term large-vessel complications (e.g., aneurysm or stenosis). Further, vascular imaging is expensive, not always covered by insurance companies for the purpose of large-vessel vasculitis care, and in some regions its use is confined to large academic hospitals. Further study of these imaging modalities for the purpose of assessing treatment response is required.
An Effective Treatment Strategy
GCA carries a substantial morbidity burden related not only to the disease itself but also to its therapy. CS, the mainstay of treatment for GCA, effectively control the symptoms of systemic inflammation and prevent acute damage (i.e., vision loss) in most patients, but generally fail to cure the disorder or induce sustained remissions. Current standards of care generally require lengthy courses of CS, on the order of a minimum of one year in most cases. Unfortunately, 40–80% of GCA patients relapse following the cessation of CS and prove to require much longer courses of these medications. The well-known adverse effects of CS—weight gain, diabetes mellitus, hypertension, osteoporotic fragility fractures, cataracts, gastrointestinal bleeding, infection, skin bruising and mood changes—are likely to be accentuated among the predominantly elderly and female GCA patient population.11
No clear alternative to long-term treatment with CS has been defined for GCA. Unfortunately, clinical trials of immunosuppressive medications, including methotrexate and tumor necrosis factor (TNF)–α antagonists, have shown mixed results or been unsuccessful.
New Targets for Therapy
The etiology of GCA remains elusive. However, research suggests that two distinct pathways driven by T helper (Th) 17 and Th1 lymphocytes, and a suppressed regulatory T-cell response contribute to disease pathogenesis.12 Whereas prednisone has been shown to correct abnormalities described in the Th17 axis of untreated patients, reports regarding the effects of CS on the upregulated Th1 response are contradictory.12,13,14 Moreover, decreased numbers of regulatory T cells are seen in the bloodstream of patients regardless of disease activity or CS treatment.14
It’s possible that a differential susceptibility exists among lymphocyte subpopulations to CS, explaining the high rate of relapse or the presence of subclinical vascular inflammation in some individuals. This theory is the subject of ongoing investigation.
The IL-6 pathway, overexpressed in GCA, represents a potential target for therapy. IL-6 has diverse biological functions depending on its target cell. During physiologic inflammatory responses, IL-6 triggers the synthesis of acute-phase proteins, promotes the transition from acute to chronic inflammation, and facilitates the development of specific immunity. IL-6 modulates the activation, proliferation and differentiation of T cells (including Th17 and regulatory T cells), and induces cells of the monocyte, endothelial and stromal lineages to acquire a “proinflammatory” phenotype.
In GCA patients, the IL-6 gene is actively transcribed within inflamed arteries, and the IL-6 concentration is elevated in peripheral circulation. Serum IL-6 levels parallel disease activity and decline with adequate CS treatment.15 Therefore, IL-6 signaling inhibition may ameliorate large-vessel vasculitis in GCA through different mechanisms that include altering upstream differentiation of autoreactive lymphocytes, promoting the generation of regulatory T cells, and/or deamplifying downstream aspects of the innate inflammatory network.
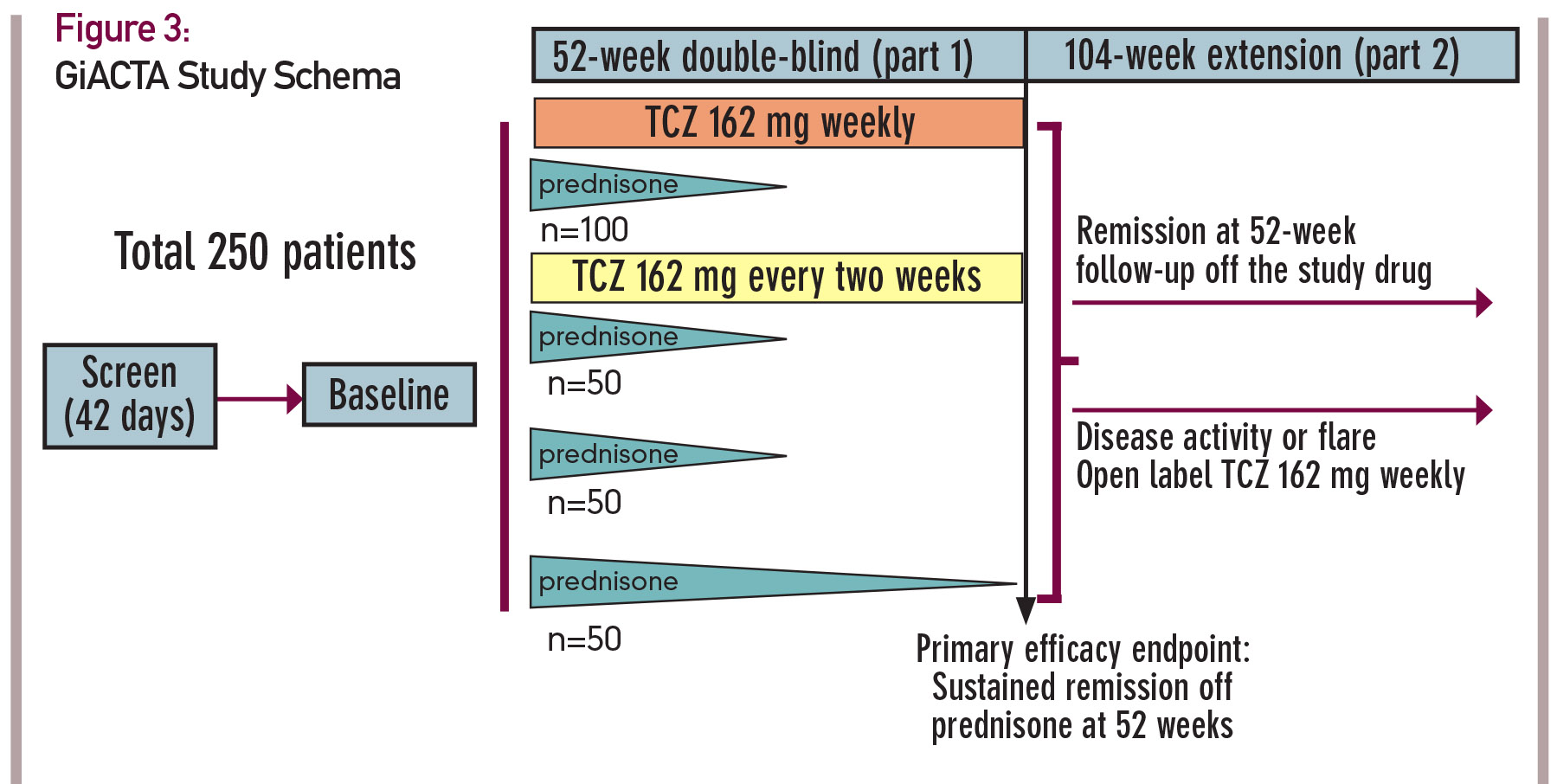
In published reports, approximately two dozen of patients with GCA have received the IL-6 receptor (IL-6R) antagonist tocilizumab (TCZ) with encouraging preliminary results.16 To rigorously evaluate the safety and efficacy of IL-6R blockade in GCA, a Phase III, multicenter, randomized, double-blind, placebo-controlled study is currently ongoing.17
In this trial, 250 patients will be enrolled and assigned to one of four treatment arms. The design of this trial, known as GiACTA, is shown in Figure 3 (see p. 23). GiACTA consists of a 52-week blinded period, followed by 104 weeks of safety extension. Two subcutaneous doses of TCZ are being compared with placebo. All patients also receive background CS therapy. Three groups follow a prespecified prednisone taper regimen over 26 weeks, and a fourth group receives a 52-week prednisone taper as monotherapy. The primary endpoint of the clinical trial is glucocorticoid-free remission at 52 weeks after randomization.
Of note, this research endeavor will also serve as the platform for important ancillary substudies that include imaging investigations (e.g., PET/CT and vascular ultrasound), mechanistic analysis and, for the first time in this disease, genome-wide gene expression profiling.
At the time of this writing, the GiACTA trial is more than 80% enrolled; 210 patients with GCA are already participating in this trial. The trial is scheduled to complete enrollment in late spring 2015.
Conclusions
At this juncture several outstanding problems need to be addressed to move the field of GCA forward. Although GCA is the most common form of primary vasculitis among adults in the U.S. and Europe, the cause of this disease is still unknown and its pathogenesis is poorly understood. Vascular inflammation in GCA can have catastrophic consequences, and standardized criteria for accurate diagnosis are lacking. Further, there is no acceptable alternative to treatment with prolonged courses of CS, which are highly toxic in this elderly population of patients.
IL-6 signaling inhibition therapy is currently being tested in a large multicenter clinical trial. The exact role of MRA/MRI, CTA and PET with respect to initial diagnosis, evaluation of treatment response, disease activity monitoring, and prediction of disease relapse and late arterial complications is to be defined. For this purpose, vascular imaging outcomes need to be factored into the design of clinical trials.
Sebastian Unizony, MD, is co-director of the Vasculitis and Glomerulonephritis Center in the Rheumatology, Allergy and Immunology Division of Massachusetts General Hospital at Harvard Medical School in Boston.
John H. Stone, MD, MPH, is director of clinical rheumatology in the Rheumatology, Allergy and Immunology Division of Massachusetts General Hospital in Boston. He is a professor of medicine at Harvard Medical School.
References
- Lee JL, Naguwa SM, Cheema GS, Gershwin ME. The geo-epidemiology of temporal (giant cell) arteritis. Clin Rev Allergy Immunol. 2008 Oct;35(1–2):88–95.
- Salvarani C, Cantini F, Boiardi L, Hunder GG. Polymyalgia rheumatica and giant-cell arteritis. N Engl J Med. 2002 Jul 25;347(4):261–271.
- Hunder GG, Bloch DA, Michel BA, et al. The American College of Rheumatology 1990 criteria for the classification of giant cell arteritis. Arthritis Rheum. 1990;33(8):1122–1128.
- Kermani TA, Schmidt J, Crowson CS, et al. Utility of erythrocyte sedimentation rate and C-reactive protein for the diagnosis of giant cell arteritis. Semin Arthritis Rheum. 2012 Jun;41(6):866–871.
- Narvaez J, Bernad B, Roig-Vilaseca D, et al. Influence of previous corticosteroid therapy on temporal artery biopsy yield in giant cell arteritis. Semin Arthritis Rheum. 2007 Aug;37(1):13–19.
- Prieto-Gonzalez S, Arguis P, Garcia-Martinez A, et al. Large vessel involvement in biopsy-proven giant cell arteritis: Prospective study in 40 newly diagnosed patients using CT angiography. Ann Rheum Dis. 2012 July;71(7):1170–1176.
- Meller J, Strutz F, Siefker U, et al. Early diagnosis and follow-up of aortitis with [(18)F]FDG PET and MRI. Eur J Nucl Med Mol Imaging. 2003 May;30(5):730–736.
- Grayson PC, Maksimowicz-McKinnon K, Clark TM, et al. Distribution of arterial lesions in Takayasu’s arteritis and giant cell arteritis. Ann Rheum Dis. 2012 Aug;71(8):1329–1334.
- Blockmans D, de Ceuninck L, Vanderschueren S, et al. Repetitive 18F-fluorodeoxyglucose positron emission tomography in giant cell arteritis: A prospective study of 35 patients. Arthritis Rheum. 2006 Feb15;55(1):131–137.
- Besson FL, Parienti JJ, Bienvenu B, et al. Diagnostic performance of (1)(8)F-fluorodeoxyglucose positron emission tomography in giant cell arteritis: A systematic review and meta-analysis. Eur J Nucl Med Mol Imaging. 2011 Sep;38(9):1764–1772.
- Proven A, Gabriel SE, Orces C, et al. Glucocorticoid therapy in giant cell arteritis: Duration and adverse outcomes. Arthritis Rheum. 2003 Oct 15;49(5):703–708.
- Deng J, Younge BR, Olshen RA, et al. Th17 and Th1 T-cell responses in giant cell arteritis. Circulation. 2010 Feb 23;121(7):906–915.
- Terrier B, Geri G, Chaara W, et al. Interleukin-21 modulates Th1 and Th17 responses in giant cell arteritis. Arthritis Rheum. 2012 Jun;64(6):2001–2011.
- Samson M, Audia S, Fraszczak J, et al. Th1 and Th17 lymphocytes expressing CD161 are implicated in giant cell arteritis and polymyalgia rheumatica pathogenesis. Arthritis Rheum. 2012 Nov;64(11):3788–3798.
- Weyand CM, Fulbright JW, Hunder GG, et al. Treatment of giant cell arteritis: Interleukin-6 as a biologic marker of disease activity. Arthritis Rheum. 2000 May;43(5):1041–1048.
- Unizony S, Stone JH, Stone JR. New treatment strategies in large-vessel vasculitis. Curr Opin Rheumatol. 2013 Jan;25(1):3–9.
- Unizony SH, Dasgupta B, Fisheleva E, et al. Design of the tocilizumab in giant cell arteritis trial. Int J Rheumatol. 2013; 2013:912562.