
Image Credit: conejota/shutterstock.com
Recognizing the need to provide guidance on the current disparate management of polymyalgia rheumatica (PMR), the American College of Rheumatology (ACR), in collaboration with the European League Against Rheumatism (EULAR), recently published the first international set of recommendations for the screening, treatment and management of PMR.1,2
Specifically, the recommendations offer guidance on the use of glucocorticoids (GCs) (e.g., initial dose, strategies for tapering off), the use of disease-modifying anti-rheumatic drugs (DMARDs) and the duration of treatment.
Acknowledging the marked, non-evidence-based variations in the use of these therapies is particularly important because of the high rates of GC-related side effects in people with PMR, according to Eric Matteson, MD, chair of rheumatology at the Mayo Clinic in Rochester, Minn., and the ACR co-principal investigator of the guideline development project.

Dr. Matteson
Studies show that approximately 85% of patients with PMR experience GC-related side effects. Further, up to 45% of patients with PMR don’t respond adequately to GCs within three to four weeks, and relapses and long-term dependency on these agents are common.1
The EULAR co-principal investigator of the guideline project, Bhaskar Dasgupta, MD, Department of Rheumatology at Southend University Hospital, Prittlewell Chase in Westcliff-on-Sea, Essex, U.K., emphasizes the need to address the variation in GC use, citing the narrow risk–benefit ratio of steroids to treat PMR and the common GC-related side effects, as well as the fact that PMR is the most common reason for the use of long-term steroid therapy.
Recommendations
The recommendations highlight evidence that supports reducing the dose and length of initial and maintenance GC therapy, as well as data on the efficacy of other agents. The recommendations are based on a systematic review of the literature of relevant studies published between January 1970 and April 2014.1
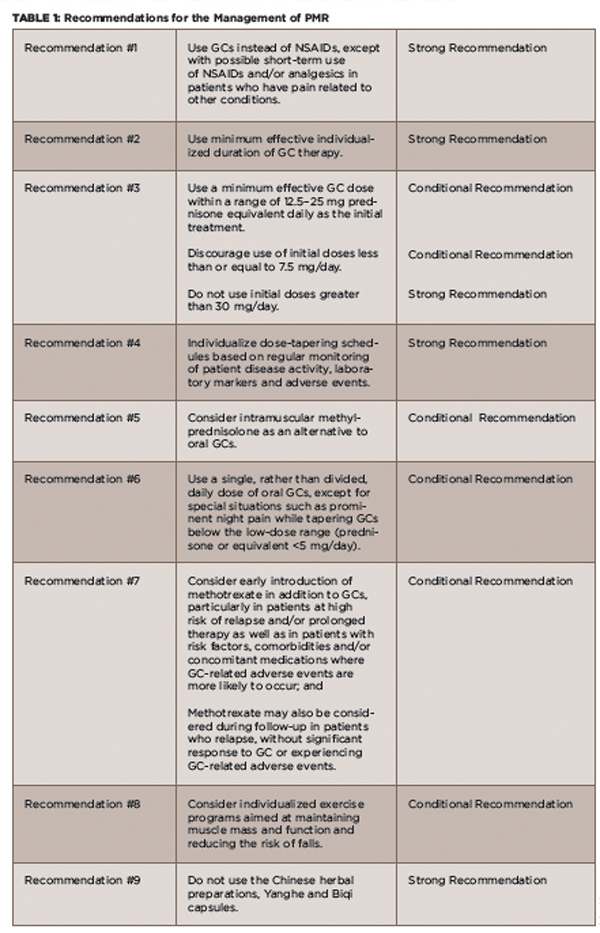
(click for larger image)
TABLE 1: Recommendations for the Management of PMR
Definitions: GC = glucocorticoid; NSAIDs = non-steroidal anti-inflammatory drugs; Strong Recommendation = Ample evidence of a large benefit with no or little risk; Conditional Recommendation = Little or modest evidence of benefit, and/or the benefit did not greatly outweigh the risks.
Source: Adapted from the ACR Press Release 20152
The multidisciplinary and international panel used the Grading of Recommendations, Assessment, Development and Evaluation (GRADE) methodology to evaluate the evidence. The direction (i.e., in favor of or against) and strength (i.e., strong or conditional) of the recommendations are based on the quality of the evidence, the balance between desirable and undesirable effects, values and preferences of the patients and clinicians, and resource use.1 (A more complete description of the methods used in this study can be found online.)

