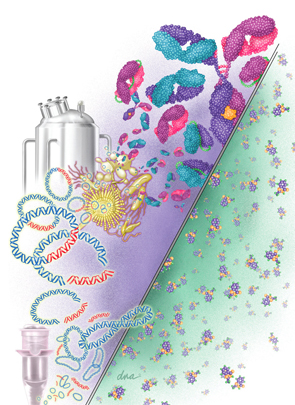
Biosimilars are much bigger and more complex on the molecular level than small-
molecule drugs. On the right is the small-molecule structure of aspirin (21 molecules). On the left is a monoclonal antibody made up of 10,000–20,000 molecules. The various biosimilar drugs depicted include monoclonal antibodies, scFv, Fab, and scFab, whereas the small-molecule drugs are identical. Also shown is a vial used for recombinant DNA procedures with plasmids as vectors and the desired fragments of DNA that are attached to the plasmids, forming recombinant plasmids. These are inserted into various living organisms, which produce the desired biosimilars in a fermentation tank.
DNA Illustrations/ScienceSource.com
On Feb. 9, the Arthritis Advisory Committee of the U.S. Food and Drug Administration (FDA) met in Washington, D.C., and recommended the approval of CT-P13, a proposed biosimilar to infliximab (Remicade), for indications including the treatment of rheumatoid arthritis, in combination with methotrexate; ankylosing spondylitis; and psoriatic arthritis. The human monoclonal antibody would be the first biosimilar available in the U.S. market to treat rheumatic diseases.
Biosimilars will offer welcome cost savings for both patients and payers. Still, some rheumatologists express concerns about the drugs, including how they will be named, fulfilled by the pharmacy, and tracked for safety and efficacy. The FDA has established two potential designations for these drugs—biosimilar and interchangeable—based on how their clinical data meet certain standards compared with the already-licensed “reference” biologic.
Sponsors must prove that the drugs are highly similar to the reference product through extensive, comparative analysis and a clinical study to assess meaningful differences between the two products. Biosimilars are biologics that are highly similar to the reference drug, but interchangeables must meet a higher standard and could be substituted for the reference drug by pharmacies without prescriber knowledge/intervention. An interchangeable must demonstrate that it produces the same clinical result as its reference product in any given patient and that the risk, in terms of safety, or diminished efficacy of alternating or switching between an interchangeable and the reference product is not greater than remaining on the reference drug. This means that the interchangeable drug may be substituted for the reference drug by a pharmacist without intervention by the prescriber. State laws govern pharmacy substitution, so it’s a complex, unfolding process.



