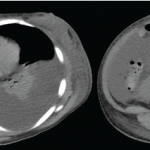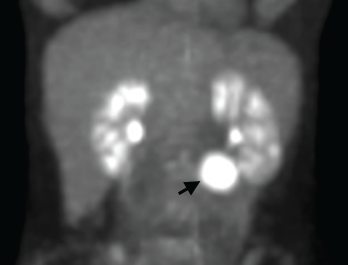
Figure 1. A positron emission tomography (PET) scan shows a 3 cm, hypermetabolic mass adjacent to the stomach.
Consulting rheumatologists often assess patients with atypical clinical presentations for the possibility of an underlying rheumatic disease. Inflammatory syndromes that are not clearly rheumatic in nature can be particularly challenging to diagnose. Here, we share the case of a young woman with a long-standing undiagnosed illness and highly elevated inflammatory markers, and describe the evaluation that led to a diagnosis of, and treatment for, unicentric Castleman disease.
Case Report
A 32-year-old woman presented to our clinic for an additional opinion. For the preceding nine years, she had experienced intermittent two- to three-month episodes of fevers, night sweats, abdominal and mid-back pain, facial rash, arthralgias and paresthesias of the extremities, without clear precipitants. Her symptoms had previously improved, in part, upon treatment with low-dose prednisone.
Her past medical history was notable for psoriasis without arthritis and an episode of unexplained anemia associated with a flare of her symptoms. Her family history included an aunt with rheumatoid arthritis and multiple relatives with thyroid disease.
The patient was healthy appearing, with normal vital signs and an unremarkable physical examination. Her complete blood count was normal. She had an elevated erythrocyte sedimentation rate (ESR) of 80 mm/hr (normal <20 mm/hr). Her C-reactive protein (CRP) was also high at 106 mg/L (normal <8 mg/L). Past laboratory records revealed similar previous elevations of ESR and CRP.
Urinalysis revealed between 1+ and 2+ proteinuria via dipstick, but was otherwise unremarkable. Serum anti-nuclear antibodies (ANA), anti-Ro (SSA) antibodies and anti-neutrophil cytoplasmic antibodies (ANCA) were absent, as were rheumatoid factor and anti-cyclic citrullinated peptide (CCP) antibodies. Complement tests were normal. Immunoglobulin concentrations, including IgG4, were normal.
An HIV serology test was negative. Flow cytometry showed a normal leukocyte subset distribution without evidence of immunodeficiency or malignancy. A test for anti-tissue transglutaminase (tTG) IgA, performed to screen for atypical presentation of celiac disease, was also negative.
We performed a positron emission tomography (PET) scan to evaluate for occult malignancy. This revealed a 3 cm hypermetabolic mass inferior to the stomach (see Figure 1, below). After a fine needle aspiration biopsy proved unrevealing, a surgeon removed the entire mass.
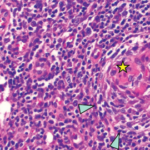
Pathological analysis demonstrated follicular hyperplasia with atretic follicles surrounded by concentric rimming of mantle zone cells. Vascular proliferation with perpendicularly penetrating vessels was also present. These morphologic features suggested Castleman lymphadenopathy (see Figure 2, opposite).
Following surgery, the patient’s inflammatory markers normalized, and her disease flares ceased. She was referred to the Castleman Disease Collaborative Network Registry (https://www.cdcn.org/accelerate), which seeks a comprehensive understanding of the pathogenesis of Castleman disease and improved treatments for people with all forms of the disease.
Discussion
Our patient presented with an atypical, episodic clinical syndrome and striking elevations in ESR and CRP. This was highly suspicious for an underlying inflammatory illness, but did not indicate a specific etiology.
Elevations in ESR and CRP can be multifactorial: The differential diagnoses for persistent highly elevated inflammatory markers include inflammatory and autoimmune rheumatic diseases, malignancy, occult infection and previously undiagnosed periodic fever syndrome or other genetic immune hyperactivation syndrome. After ruling out serologic evidence indicating an autoimmune disease, rheumatologists can assess for these other possibilities, with the input of additional specialists as necessary.
We suggest FDG-PET scanning as a potential tool for these evaluations, such as we used for our patient. Areas of increased metabolic activity detected by these scans suggest foci of inflammation that can provide diagnostic clues.1
An additional option is referral for whole exome sequencing to assess for any underlying hyperinflammatory mutation; the diagnostic capabilities of such precision medicine techniques will likely expand in the years to come.2