Gout affects more than 9.2 million adults in the U.S. and is the most common form of inflammatory arthritis. This condition and its complications are painful and potentially disabling with varying risk factors. It is characterized by symptoms that are usually sudden, with intense episodes of painful swelling in one or more joints, most often in the big toe. However, gout is more than just flares that come and go. Without adequate management of gout, urate can build up, leading to tophi and joint destruction. Fortunately, strategies exist to manage symptoms and prevent complications, such as flares and tophi.
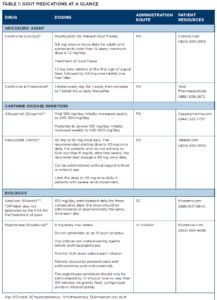
The 2020 ACR Guideline on the Management of Gout, which serves as the starting point for the clinical management of gout patients is discussed.1 And a pullout guide to the drugs available to treat the disease is provided (see Table 1 below).
What the Expert Says
Recently, The Rheumatologist interviewed Dr. Neogi, an international expert in the field, about the clinical management of gout. Below, we discuss the updated ACR Guideline on the Management of Gout, clinical pearls, strategies to avoid common mistakes and recent research around pegloticase. Tuhina Neogi, MD, PhD, is chief of rheumatology and a professor of medicine at Boston University School of Medicine, and a professor of epidemiology at Boston University School of Public Health. Her research primarily focuses on gout and osteoarthritis. She has been published in more than 250 peer-reviewed publications and has received funding from the National Institutes of Health for her research since 2003.
Dr. Neogi helped lead the process to update the ACR Guideline on the Management of Gout and has been a featured speaker at many ACR/ARP annual meetings. She has served on national committeesfor the ACR, was past chair of the U.S. Food & Drug Administration (FDA) Arthritis Advisory Committee, and has served on two boards of the international societies: Osteoarthritis Research Society International (OARSI) and Gout, Hyperuricemia, and Crystal-associated Diseases Network (G-CAN).
In addition to providing clinical care, conducting research and teaching, one of Dr. Neogi’s key roles is acting as a mentor to trainees and junior faculty. Her research and mentorship have been recognized with prestigious awards, including the 2016 Robert Dawson Evans Research Mentoring Award and the 2014 ACR Henry Kunkel Young Investigator Award.
The Rheumatologist (TR): You served as co-lead when the gout management guideline was revamped. Can you tell us some of the new recommendations?
Dr. Neogi: One key difference from the 2012 ACR gout treatment guideline is that allopurinol is now strongly recommended as first-line therapy over other agents, including in individuals with renal insufficiency. In 2012, both allopurinol and febuxostat were considered first-line agents, but the methodology used for the 2012 guideline was not able to take cost into consideration. Additionally, the cardiovascular safety questions surrounding febuxostat were not present during the development of the 2012 guideline.
The 2020 ACR Guideline on the Management of Gout made a conditional recommendation regarding the use of febuxostat in those with a history of cardiovascular disease or new cardiovascular event due to the FDA’s Boxed Warning that arose due to the CARES trial results.2 In light of newer cardiovascular safety data from the FAST trial, the data regarding febuxostat are more reassuring.3
Although not a specific difference per se from the prior guideline, the ACR Guideline on the Management of Gout reinforces a treat-to-target management strategy that is strongly recommended, with a serum urate target of <6 mg/dL due to additional interim data that had been published since 2012, providing moderate- and high-quality evidence for these recommendations. However, lower serum urate targets for those with more severe disease (e.g., those with tophi) were not specified given that clinical trials have not specifically evaluated lower thresholds. Nonetheless, observational data support lower thresholds for those with tophi to hasten tophus resolution and reduce the frequency of flares.
With the ACR Guideline on the Management of Gout, indications for urate-lowering therapy initiation were expanded to include individuals with radiographic evidence of bony damage attributable to gout (strong recommendation). There were also new conditional recommendations, meaning these warrant additional shared decision-making discussions, such as initiation of urate-lowering therapy in patients with infrequent flares or in those with a first flare and marked hyperuricemia (i.e., serum urate >9 mg/dL).
TR: What are some of the new therapy recommendations in gout management?
Dr. Neogi: Since 2012, lesinurad combined with a xanthine oxidase inhibitor had been FDA approved but had since been removed from the market; therefore, specific recommendations regarding the use of lesinurad could not be made. Nonetheless, the lesinurad trial data provided additional information to the evidence report regarding efficacy of this mechanism of action.4,5
For management of gout flares, if anti-inflammatory therapies are ineffective, poorly tolerated or contraindicated, use of interleukin (IL) 1 inhibition was conditionally recommended, providing another option for management of this exquisitely painful manifestation of gout.
TR: There’s been recent research around pegloticase. What are your thoughts on using it in the clinical management of gout?
Dr. Neogi: Pegloticase is a particularly important option for patients in whom oral urate-lowering therapy is insufficient in managing their gout. In the 2020 ACR Guideline on the Management of Gout, pegloticase was strongly recommended for patients with gout for whom xanthine oxidase inhibition, uricosuric therapy and other interventions have failed to achieve the serum urate target and who continue to have frequent gout flares (≥2 flares/year) or who have non-resolving subcutaneous tophi.
Newer data have been reported in small studies demonstrating the ability to diminish the risk for losing the response to pegloticase due to antibodies which raise the risk of infusion reaction with the use of concomitant immunosuppressive agents.6,7 Those data were not available at the time the 2020 ACR Guideline on the Management of Gout was being developed. As more data become available, they will be incorporated into the next update.
TR: What do you believe are the essentials in interacting with gout patients?
Dr. Neogi: Education and clear communication are essential. Having patients understand the disease and its management is key. It is helpful for patients to understand gout as being driven by their body’s response to excess levels of urate, that the main treatment is to lower serum urate and that anti-inflammatory therapy is used to help prevent flares when initiating urate-lowering therapy, as well as to treat gout flares. The most important concept is to understand that urate-lowering therapy is foundational to getting gout under control and that part of the journey is returning for regular follow-up visits so the medication can be titrated to the level their body needs to get the gout under control.
TR: What are the most common mistakes you see in management of gout?
Dr. Neogi: Unfortunately, there are lots of mistakes in the management of gout! There are three common mistakes I see in terms of urate-lowering therapy.
- People are not started on urate-lowering therapy at all when they have a clear indication, such as frequent flares or tophi. Often in such scenarios, people are just managed symptomatically for their flare episodes, but that doesn’t address the underlying problem and can lead to more severe gout down the road that may be more difficult to manage.
- Another common mistake is that someone gets started on urate-lowering therapy (e.g., with allopurinol) but then the dose is never titrated up to achieve a serum urate of <6 mg/dL.
- People get started on allopurinol 300 mg/day without prophylaxis, rather than at a low dose and titrated up. So when the individuals experience more flares due to that strategy, they think the physician doesn’t know what they are doing or the medication doesn’t work because their gout is now worse.
- For flare management, a common problem is that patients are often not provided an appropriate dose or duration of the anti-inflammatory therapy.
TR: What are some tips for the clinical management of gout?
Dr. Neogi: When someone is diagnosed with gout and has an indication for starting urate-lowering therapy, allopurinol should be started at a low dose—100 mg/day in most people; 50 mg/day in people with chronic kidney disease (CKD) stage 4 or worse—to mitigate the risk of allopurinol hypersensitivity syndrome and to also lower the risk of flares in this initial phase of therapy.
Prophylaxis should be provided at the time of urate-lowering therapy initiation.
The dose of the urate-lowering medication needs to be up-titrated guided by serum urate; this can be done every few weeks. It’s important to avoid titration inertia. It’s also important to realize that it may take one to two years before flares subside when using oral urate-lowering therapy. The key is to maintain serum urate at <6 mg/dL, and escalate therapy as appropriate if the target urate is not achieved and there is ongoing disease activity.
Patients should also be given appropriate anti-inflammatory therapy for gout flare management to be kept with them at all times—a so-called medication in-pocket strategy—so they can initiate gout flare treatment the moment a flare starts rather than waiting for a prescription to be called in or needing to be seen in person, which can take days. An early start to anti-inflammatory therapy can often minimize the duration and severity of the flare.
Mary Choy, PharmD, BCGP, FASHP, is a medical writer and editor living in New York City. Dr. Choy is director of pharmacy practice at the New York State Council of Health-system Pharmacists. She is also the co-author of Healthcare Heroes: The Medical Careers Guide.
References
- FitzGerald, JD, Dalbeth N, Mikuls T, et al. 2020 American College of Rheumatology Guideline for the Management of Gout. Arthritis Care Res (Hoboken). 2020 Jun;72(6):744–760.
- White WB, Saag KG, Becker MA, et al. Cardiovascular safety of febuxostat or allopurinol in patients with gout. N Engl J Med. 2018 Mar 29;378(13):1200–1210.
- Mackenzie IS, Ford I, Nuki G, et al. Long-term cardiovascular safety of febuxostat compared with allopurinol in patients with gout (FAST): A multicentre, prospective, randomised, open-label, non-inferiority trial. Lancet. 2020 Nov 28;396(10264):1745–1757.
- Dalbeth N, Jones G, Terkeltaub R, et al. Lesinurad, a selective uric acid reabsorption inhibitor, in combination with febuxostat in patients with tophaceous gout: Findings of a phase III clinical trial. Arthritis Rheumatol. 2017;69:1903–1913.
- Saag KG, Fitz-Patrick D, Kopicko J, et al. Lesinurad combined with allopurinol: A randomized, double-blind, placebocontrolled study in gout patients with an inadequate response to standard-of-care allopurinol (a US-based study). Arthritis Rheumatol. 2017;69:203–212.
- Khanna PP, Khanna D, Cutter G, et al. Reducing immunogenicity of pegloticase with concomitant use of mycophenolate mofetil in patients with refractory gout: A phase II, randomized, doubleblind, placebo-controlled trial. Arthritis Rheumatol. 2021 Aug;73(8):1523–1532.
- Botson JK, Tesser JRP, Bennett R, et al. Pegloticase in combination with methotrexate in patients with uncontrolled gout: A multicenter, open-label study (MIRROR). J Rheumatol. 2021 May;48(5):767–774.
- U.S. Food & Drug Administration. Colcrys prescribing information. 2015 Dec. https://tinyurl.com/2p9bna2c.
- Label: Probenecid and colchicine tablet. DailyMed. U.S. National Library of Medicine. https://tinyurl.com/2p87y75a.
- U.S. Food & Drug Administration. Zyloprim (allopurinol) prescribing information. 2018 Dec. https://tinyurl.com/yyfktdmf.
- U.S. Food & Drug Administration. Uloric prescribing information. 2019 Feb. https://tinyurl.com/yujpax4p.
- Khanna A, Khanna PP, FitzGerald JD, et al. 2012 American College of Rheumatology Guidelines for Management of Gout. Part 2: Therapy and anti-inflammatory prophylaxis of acute gouty arthritis. Arthritis Care Res (Hoboken). 2012 Oct;64(10):1447–1461.
- U.S. Food & Drug Administration. Kineret prescribing information. 2018 Jun. https://tinyurl.com/3u4sx7uu.
- U.S. Food & Drug Administration. Krystexxa prescribing information. 2018 Jul. https://tinyurl.com/2p86m7t6.