 The axial phenotype of psoriatic arthritis (axPsA) is an excellent example of a major controversy in rheumatology that has become the focus of attention because of the emergence of new therapies with different mechanisms of action for alleviating joint inflammation. It was first described in 1961 but, until recently, it has largely remained under the radar of many rheumatologists, being regarded as a forme fruste of ankylosing spondylitis (AS).1 This has been reinforced by its association with human leukocyte antigen B27 (HLA-B27) and radiographic findings in the spine and sacroiliac joints (SIJ) resembling those observed in AS.
The axial phenotype of psoriatic arthritis (axPsA) is an excellent example of a major controversy in rheumatology that has become the focus of attention because of the emergence of new therapies with different mechanisms of action for alleviating joint inflammation. It was first described in 1961 but, until recently, it has largely remained under the radar of many rheumatologists, being regarded as a forme fruste of ankylosing spondylitis (AS).1 This has been reinforced by its association with human leukocyte antigen B27 (HLA-B27) and radiographic findings in the spine and sacroiliac joints (SIJ) resembling those observed in AS.
A resurgence of interest has been sparked by emerging data from clinical trials that axPsA and AS may respond differentially to therapeutic agents. In particular, although therapies targeting interleukin (IL) 23, such as ustekinumab and risankizumab, were not effective in trials of AS, post hoc analyses of PsA trials have reported efficacy in subgroups of patients who had spinal symptoms, in addition to their peripheral joint inflammation, and were deemed to have axial inflammation (axPsA), per the opinion of the local investigator.2-5
This has generated controversy: Are these differences in response to treatment real, or do they reflect the lack of an evidence-based case definition for axPsA? Are some patients being misdiagnosed as having axPsA, when their back pain actually has another cause? Also, outcome measures that lack specificity for axial inflammation may actually reflect improvement in peripheral joint inflammation.6 For example, patients reporting improvement in their peripheral joint symptoms on questionnaires, such as the Bath Ankylosing Spondylitis Disease Activity Index (BASDAI), may also report improvement in spinal symptoms as a bystander effect.
A major international study aimed at providing the evidence for a new case definition is underway, the Axial Involvement in Psoriatic Arthritis Cohort (AXIS), but an expert consensus has already emerged that any such definition must include radiographic evidence of axPsA.7,8
In my overview of this topic, I outline what is currently known about the imaging of axPsA, how imaging may complement clinical assessment, the pitfalls of imaging assessments typically used in routine evaluation of axPsA and how imaging can be used to help define patients with axPsA.
axPsA Imaging
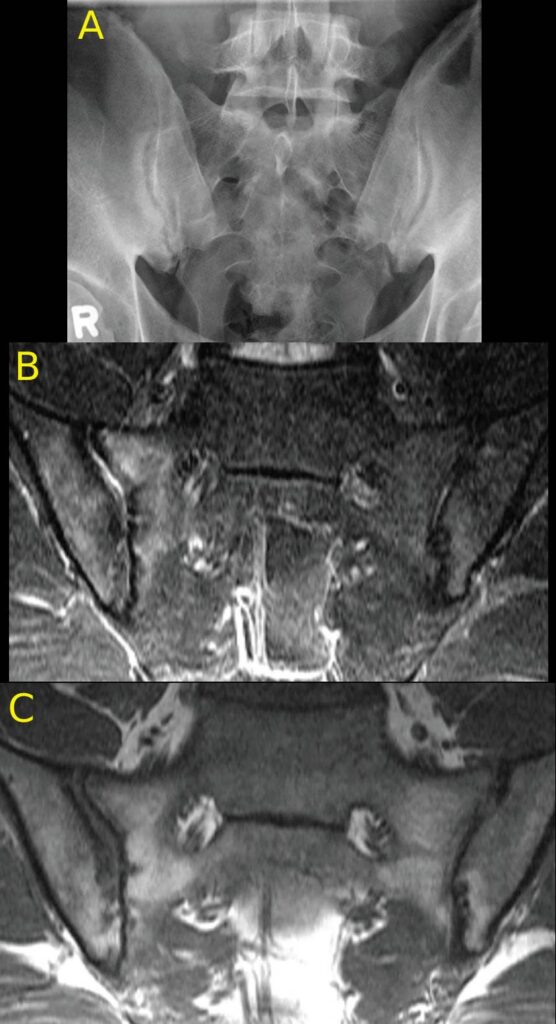
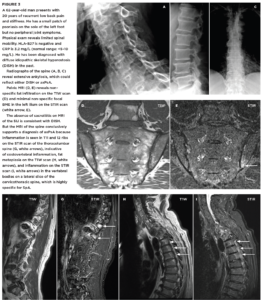
FIGURES 1a, 1b and 1C (click to enlarge). A 33-year-old male nurse presents with lower back pain of nine months’ duration that has inflammatory characteristics. He has mild scalp psoriasis and oligoarticular synovitis that are well controlled on 20 mg of methotrexate by mouth weekly. The physical exam is unremarkable. HLA-B27 is negative and C-reactive protein (CRP) is 18 mg/L (normal range: <5–10 mg/L).
Figure A. Mild iliac sclerosis and small iliac erosions at the inferior portion of the right SIJ are evident on the radiograph of the sacroiliac joints. The left SIJ is normal.
Figure B. STIR MRI scan of the pelvis demonstrates bone marrow edema in the right sacrum and ilium.
Figure C. The T1W MRI scan of the pelvis demonstrates erosion of the right and left iliac cortex, post-inflammatory fat metaplasia in the right sacrum (white asterisk) and fat metaplasia in an erosion cavity.
Although the radiographic findings are unilateral and subtle, the MRI findings include both active and structural lesions typical of axial inflammation.
Surprisingly little is known about imaging of the SIJ and spine, and this is almost entirely confined to conventional radiographic (CR) observations. Studies conducted over two decades ago compared different groups of patients with AS defined by their extra-articular manifestations and reported a variety of radiographic features that were considered characteristic of patients with axPsA.9 These included asymmetric or unilateral sacroiliitis (see Figure 1A); paravertebral ossification extending between the vertebral cortices of adjacent vertebrae; and exuberant and non-marginal syndesmophytes, as opposed to the slender vertical syndesmophytes observed in AS.
Other reports have described a tendency for focal involvement of the spine, especially the cervical spine, as opposed to the more typical progressive involvement of lumbar to cervical segments characteristic of AS, and involvement of the atlanto-axial joint similar to that observed in rheumatoid arthritis.10-12 Spinal abnormalities in the absence of SIJ involvement have also been described.13,14
Superiority of MRI
We have a paucity of data from magnetic resonance imaging (MRI) evaluation, which has the capacity to assess inflammatory as well as structural lesions that cannot be observed on CR. Fat suppressed but water-sensitive MRI sequences, such as short tau inversion recovery (STIR), are required to detect bone marrow edema (BME) associated with inflammation in the bone marrow of the SIJ and spine (see Figure 1B). The adult bone marrow of the SIJ and spine contains fat, and the signal from fat must be suppressed to visualize the BME. The T1-weighted sequence is fat sensitive, and bone is dark on both sequences; this sequence provides good visualization of the subchondral bone and bone marrow interface for detection of structural lesions, such as erosion and fat metaplasia (see Figure 1C).
When using computed tomography (CT) as the gold standard, MRI is far more sensitive and specific than CR for the detection of erosion and ankylosis.15 When using expert rheumatologist diagnosis as the gold standard, MRI is again far superior to CR for diagnostic accuracy.16
The Assessment of SpondyloArthritis International Society (ASAS) recently reported an international consensus of standardized MRI lesion definitions for the SIJ and spine.17,18 These should be systematically applied to studies evaluating MRI lesions in axPsA.
A recent comparison of MRI scans of the SIJ from an inception cohort of patients presenting with undiagnosed back pain and one of psoriasis, colitis, acute anterior uveitis and diagnosed by a rheumatologist with axSpA did not demonstrate any significant differences in distribution or frequencies of inflammatory or structural lesions in the SIJ.19
Case-based observations in the spine have demonstrated examples of extensive focal inflammation in the spine affecting entire vertebrae with limited or no involvement of the SIJ (see Figure 2). MRI may be particularly helpful in the setting of extensive spinal ankylosis resembling diffuse idiopathic skeletal hyperostosis that may appear similar to axPsA on conventional radiography because both conditions may lack involvement of the SIJ. MRI features that are especially helpful in pointing toward axPsA include bone marrow edema and/or fat metaplasia in the costovertebral and/or costotransverse joints (see Figure 3).
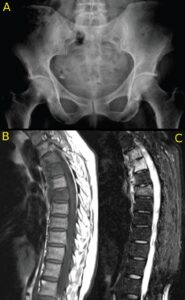
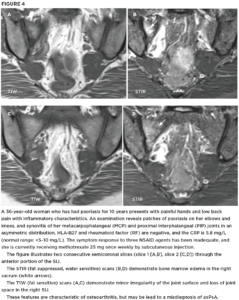
Figures 2A, 2B and 2C (click to enlarge). A 59-year-old woman presents with a three-month duration of interscapular pain initially thought to be due to osteomyelitis. She had image-guided biopsy of the T7 vertebra, but nothing was found. She received intermittent courses of antibiotics but continued to have interscapular pain. A rheumatologist noted that over the past decade she had mild psoriasis affecting her elbows and knees and not requiring continuous therapy. She was started on a TNF inhibitor, and her symptoms completely resolved.
Figure A. The pelvic radiograph is normal.
Figure B. The T1W MRI scan of the thoracic spine demonstrates partial collapse of the T7 upper endplate (arrow) and loss of the marrow fat signal in T4, T5 and T7 vertebrae.
C. The STIR scan demonstrates osteitis affecting the entire T4, T5, T7 vertebrae with soft tissue inflammation anterior to these vertebrae.
A significant challenge for clinicians evaluating patients presenting with undiagnosed back pain as well as psoriasis and/or PsA is the lack of correlation between inflammatory features of back pain and findings pointing to axSpA on radiography or MRI.20-22 In fact, reports have shown that 33–55% of patients with radiographic abnormalities have no back symptoms.14,23,24
In view of these reports, plus observations that up to 70% of patients with long-standing PsA may develop axial manifestations, it would be appropriate to assess any such patient presenting with back pain using MRI to provide the most accurate diagnosis, especially if the symptoms are severe and inadequately controlled by non-steroidal anti-inflammatory drugs.23
Certain therapies commonly used in PsA, such as apremilast, are not effective for axial manifestations, and agents with demonstrable efficacy for axial inflammation, at least in the setting of axSpA, such as those targeting tumor necrosis factor (TNF) or IL-17, would be preferable.25
2 Phenotypes of axPsA
An important consideration in the interpretation of imaging for axPsA is reports that support the existence of two phenotypes of axPsA.24,26 The first has its onset early in life and resembles AS, with a high frequency of HLA-B27 (80–90%), symmetrical involvement of the SIJ on imaging and spondylitis that ascends from lumbar to cervical segments. These patients have psoriasis but few peripheral manifestations in joints and entheses.
The second phenotype includes more women; becomes evident from the fourth decade of life; has a much lower frequency of HLA-B27 (20–40%); high prevalence of peripheral synovitis and enthesitis; more subtle imaging features, such as asymmetric or unilateral sacroiliitis; and sporadic findings in the spine in the absence of sacroiliitis, such as syndesmophytes. It is important to note that reliability for detection of low grades of sacroiliitis on radiography is poor, even for experienced musculoskeletal radiologists and even after extensive training and calibration.27,28
Degenerative and post-partum changes in the SIJ on both radiographs and MRI scans may resemble those observed in axSpA.29 Focal BME and fat metaplasia involving the anterior aspect of the SIJ is a common finding in osteoarthritis (see Figure 4). Degenerative disc disease may be associated with BME and fat metaplasia at vertebral corners and endplates. The shape and direction of bone growth of syndesmophytes may also overlap with degenerative disease and is insufficiently discriminatory for axPsA.
These imaging findings raise serious questions regarding the validity of the construct defining the second phenotype of axPsA. How can imaging be used to further define this construct? In a recent report from the ASAS-MRI working group, experts in MRI interpretation of axSpA used a data driven methodology to derive quantitative MRI definitions for a positive MRI with ≥95% specificity for inflammatory or structural MRI lesions typical of axSpA, as well as ≥95% positive predictive validity for rheumatologist diagnosis of axSpA.30 For inflammation typical of axSpA this was BME in ≥4 SIJ quadrants; for structural lesions this was erosion in ≥3 SIJ quadrants or fat lesion in ≥5 SIJ quadrants. This methodology could be applied to define positive imaging for axPsA so that it can be widely applied accurately and reliably.
Despite the low cost and widespread availability of CR, the future of imaging in axPsA is really MRI because the SIJ is a complex joint that requires imaging in three planes. Recent advances, such as the U.S. Food & Drug Administration approved “Bone MRI” technology, permit comparable detection of structural lesions to a CT scan. It is incumbent on rheumatologists to acquire sufficient expertise that they can communicate in the language of MRI with their radiology colleagues in the best interests of their patients. Online resources are available that can help rheumatologists achieve this goal.
 Walter P. Maksymowych, FRCP(C), is chief medical officer of CARE Arthritis Ltd., Alberta, Canada. He is also a professor and medical scientist in the Department of Medicine, Division of Rheumatology at the University of Alberta. He is the 2012 recipient of the Distinguished Investigator Award from the Canadian Rheumatology Association.
Walter P. Maksymowych, FRCP(C), is chief medical officer of CARE Arthritis Ltd., Alberta, Canada. He is also a professor and medical scientist in the Department of Medicine, Division of Rheumatology at the University of Alberta. He is the 2012 recipient of the Distinguished Investigator Award from the Canadian Rheumatology Association.
Disclosures
Dr. Maksymowych has acted as a paid consultant and participated in advisory boards for AbbVie, Boehringer Ingelheim, Celgene, Eli Lilly and Company, Galapagos, Janssen, Novartis, Pfizer and UCB; received research and/or educational grants from AbbVie, Novartis, Pfizer and UCB; and received speaker fees from AbbVie, Janssen, Novartis, Pfizer and UCB.
References
- Wright V. Psoriatic arthritis. A comparative radiographic study of rheumatoid arthritis and arthritis associated with psoriasis. Ann Rheum Dis. 1961 Jun;20(2):123–132.
- Baeten D, Østergaard M, Wei JCC, et al. Risankizumab, an IL-23 inhibitor, for ankylosing spondylitis: Results of a randomised, double-blind, placebo-controlled, proof-of-concept, dose-finding phase 2 study. Ann Rheum Dis. 2018 Sep;77(9):1295–1302.
- Deodhar A, Gensler LS, Sieper J, et al. Three multicenter, randomized, double-blind, placebo-controlled studies evaluating the efficacy and safety of ustekinumab in axial spondyloarthritis. Arthritis Rheumatol. 2019 Feb;71(2):258–270.
- Helliwell PS, Gladman DD, Chakravarty SD, et al. Effects of ustekinumab on spondylitis-associated endpoints in TNFinaïve active psoriatic arthritis patients with physician-reported spondylitis: Pooled results from two phase 3, randomised, controlled trials. RMD Open. 2020 Feb;6(1):e001149.
- Mease PJ, Helliwell PS, Gladman DD, et al. Efficacy of guselkumab on axial involvement in patients with active psoriatic arthritis and sacroiliitis: a post-hoc analysis of the phase 3 DISCOVER-1 and DISCOVER-2 studies. Lancet Rheumat. 2021 Oct 1;3(10):e715–723.
- Braun J, Landewé RBM. No efficacy of anti-IL-23 therapy for axial spondyloarthritis in randomised controlled trials but in posthoc analyses of psoriatic arthritis-related ‘physician-reported spondylitis’? Ann Rheum Dis. 2021 Oct 16;annrheumdis-2021-221422. Online ahead of print.
- Poddubnyy D. Axial involvement in psoriatic arthritis cohort (AXIS) [NCT04434885]. ClinicalTrials.gov. 2020 Jun 17.
- Goel N, FitzGerald O, Gladman DD, et al. GRAPPA 2018 project report. J Rheumatol. 2019 Jun;95(suppl):54–57.
- Helliwell PS, Hickling P, Wright V. Do the radiological changes of classic ankylosing spondylitis differ from the changes found in the spondylitis associated with inflammatory bowel disease, psoriasis, and reactive arthritis? Ann Rheum Dis. 1998 Mar;57(3):135–140.
- Salvarani C, Macchioni P, Cremonesi T, et al. The cervical spine in patients with psoriatic arthritis: A clinical, radiological and immunogenetic study. Ann Rheum Dis. 1992 Jan;51(1):73–77.
- Queiro R, Sarasqueta C, Torre JC, et al. Prevalence and predictors of cervical involvement in psoriatic spondyloarthropathy. J Clin Rheumatol. 2002 Feb;8(1):23–29.
- Sallés M, Clavaguera T, Mínguez S, et al. Atlantoaxial rotatory dislocation in a patient with psoriatic spondyloarthritis. Joint Bone Spine. 2019 Jul;86(4):519–521.
- Helliwell PS. Axial disease in psoriatic arthritis. Rheumatology (Oxford). 2020 Jun;59(6):1193–1195.
- Jadon DR, Sengupta R, Nightingale A, et al. Axial Disease in Psoriatic Arthritis study: Defining the clinical and radiographic phenotype of psoriatic spondyloarthritis. Ann Rheum Dis. 2017 Apr;76(4):701–707.
- Diekhoff T, Hermann K-GA, Greese J, et al. Comparison of MRI with radiography for detecting structural lesions of the sacroiliac joint using CT as standard of reference: Results from the SIMACT study. Ann Rheum Dis. 2017 Sep;76(9):1502–1508.
- Diekhoff T, Eshed I, Radny F, et al. Choose wisely: Imaging for diagnosis of axial spondyloarthritis. Ann Rheum Dis. 2021 Feb;81(2):237–242.
- Maksymowych WP, Lambert RG, Østergaard M, et al. MRI lesions in the sacroiliac joints of patients with spondyloarthritis: An update of definitions and validation by the ASAS MRI working group. Ann Rheum Dis. 2019 Nov;78(11):1550–1558.
- Maksymowych WP, Eshed I, Machado PM, et al. Consensus Definitions for MRI Lesions in the Spine of Patients with Axial Spondyloarthritis: First Analysis from the Assessments in Spondyloarthritis International Society Classification Cohort. Ann Rheum Dis. 2020 Jun;79(suppl 1):0317.
- Maksymowych WP, Weber U, Chan J, et al. Does imaging of the sacroiliac joint differ in patients presenting with undiagnosed back pain and psoriasis, acute anterior uveitis, and colitis: An inception cohort study. Ann Rheum Dis. 2021 Jun;80(suppl 1):224.
- Battistone MJ, Manaster BJ, Reda DJ, et al. The prevalence of sacroilitis in psoriatic arthritis: New perspectives from a large, multicenter cohort. A Department of
Veterans Affairs Cooperative Study. Skeletal Radiol. 1999 Apr;28(4):196–201. - Yap KS, Ye JY, Li S, et al. Back pain in psoriatic arthritis: Defining prevalence, characteristics and performance of inflammatory back pain criteria in psoriatic arthritis. Ann Rheum Dis. 2018 Nov;77(11):1573–1577.
- Williamson L, Dockerty JL, Dalbeth N, et al. Clinical assessment of sacroiliitis and HLA-B27 are poor predictors of sacroiliitis diagnosed by magnetic resonance imaging in psoriatic arthritis. Rheumatology (Oxford). 2004 Jan;43(1):85–88.
- Gladman DD. Axial disease in psoriatic arthritis. Curr Rheumatol Rep. 2007 Dec;9(6):455–460.
- Feld J, Ye JY, Chandran V, et al. Is axial psoriatic arthritis distinct from ankylosing spondylitis with and without concomitant psoriasis? Rheumatology (Oxford). 2019 Jun;59(6):1340–1346.
- Taylor PC, van der Heijde D, Landewé R, et al. A phase III randomized study of apremilast, an oral phosphodiesterase 4 inhibitor, for active ankylosing spondylitis. J Rheumatol. 2021 Aug;48(8):1259–1267.
- Helliwell PS. Axial disease in psoriatic arthritis. Rheumatology (Oxford). 2020 Jun 1;59(6):1193–1195.
- Christiansen AA, Hendricks O, Kuettel D, et al. Limited reliability of radiographic assessment of sacroiliac joints in patients with suspected early spondyloarthritis. J Rheumatol. 2017 Jan;44(1):70–77.
- van Tubergen A, Heuft-Dorenbosch L, Schulpen G, et al. Radiographic assessment of sacroiliitis by radiologists and rheumatologists: Does training improve quality? Ann Rheum Dis. 2003 Jun;6(6):519–525.
- Hoballah A, Lukas C, Leplat C, et al. MRI of sacroiliac joints for the diagnosis of axial SpA: Prevalence of inflammatory and structural lesions in nulliparous, early postpartum and late postpartum women. Ann Rheum Dis. 2020 Aug;79(8):1063–1069.
- Maksymowych WP, Lambert RG, Baraliakos X, et al. Data-driven definitions for active and structural MRI lesions in the sacroiliac joint in spondyloarthritis and their predictive utility. Rheumatology (Oxford). 2021 Oct 2;60(10):4778–4789.