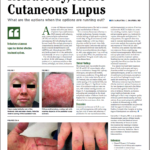
 Results from a phase 2 trial of patients with cutaneous lupus erythematosus revealed that treatment with litifilimab was superior to placebo for reducing skin disease activity at 16 weeks. The study focused on cutaneous lupus erythematosus, an autoimmune disease that can occur in the presence or absence of systemic lupus erythematosus (SLE). The study by Victoria P. Werth, MD, chief of dermatology at the Philadelphia Veterans Association Hospital, and colleagues, was published in the New England Journal of Medicine in July 2022.1
Results from a phase 2 trial of patients with cutaneous lupus erythematosus revealed that treatment with litifilimab was superior to placebo for reducing skin disease activity at 16 weeks. The study focused on cutaneous lupus erythematosus, an autoimmune disease that can occur in the presence or absence of systemic lupus erythematosus (SLE). The study by Victoria P. Werth, MD, chief of dermatology at the Philadelphia Veterans Association Hospital, and colleagues, was published in the New England Journal of Medicine in July 2022.1
Cutaneous lupus erythematosus can cause irreversible damage and disfigurement, impair quality of life and is associated with depression, anxiety and fatigue. Cutaneous lupus erythematosus manifests in several major subtypes: chronic, subacute and acute. Most patients with subacute or chronic cutaneous lupus erythematosus will not develop SLE.2
Methods
The randomized, placebo-controlled trial included 132 patients with active, histologically confirmed cutaneous lupus erythematosus with or without SLE manifestations. The investigators measured skin disease activity using the Cutaneous Lupus Erythematosus Disease Area and Severity Index-Activity (CLASI-A) score and evaluated the efficacy of litifilimab. Litifilimab is a humanized monoclonal antibody that binds blood dendritic cell antigen 2 (BDCA2), which is a receptor exclusively expressed on plasmacytoid dendritic cells. Patients were randomized receive placebo (n=33), 50 mg of litifilimab (n=26), 150 mg of litifilimab (n=25) and 450 mg of litifilimab (n=4).
Although approximately 90% of patients received concomitant background therapy, including oral glucocorticoids (prednisone at a dose ≤15 mg per day or equivalent), antimalarial drugs and immunosuppressant agents, most trial participants had moderate to severe disease with mean CLASI-A scores of 15.2 for the placebo group, 18.4 for the 50 mg litifilimab group, 16.5 for the 150 mg litifilimab group and 16.5 for the 450 mg litifilimab at baseline group.
The Results
At week 16, treatment with litifilimab improved CLASI-A scores compared with placebo. The difference in the change from baseline compared with placebo was -24.3 percentage points (95% confidence interval [CI], -43.7 to -4.9) for 50 mg of litifilimab, -33.4 percentage points (95% CI, -52.7 to -14.1) for 150 mg of litifilimab and -28.0 percentage points (95% CI, -44.6 to -11.4) for 450 mg of litifilimab. The investigators used the least squares mean changes in the primary analysis of a best-fitting, dose-response model across the three drug-dose levels and placebo, finding a significant effect.
The researchers found most of the secondary end points did not support the results of the primary analysis. These secondary end points included a decrease of at least 50% from baseline in the CLASI-A score at weeks 12 and 16, the percent change in baseline in the CLASI-A score at week 12, reductions from baseline in the CLASI-A score of at least four points at weeks 12 and 16 and of at least seven points at weeks 12 and 16, and changes in pharmacokinetic measures.