(Reuters Health)—A once-daily 15 mg dose of upadacitinib for rheumatoid arthritis (RA) has similar rates of malignancies, serious infections, major adverse cardiovascular events, and venous thromboembolic events as other Janus kinase inhibitors (jakinibs), results from phase 3 clinical trials suggest. Researchers examined data on treatment emergent adverse events among patients taking upadacitinib in five randomized…
Search results for: Jakinibs

The Great Debate: Should Jakinibs Be Used Before Biologics after Methotrexate Failure in RA?
ACR CONVERGENCE 2020—In many ways, the current plethora of treatment options for rheumatoid arthritis patients represents an embarrassment of riches. However, while many therapeutics approved by the U.S. Food & Drug Administration (FDA) are available, knowing the order in which to try these medications with patients can be quite challenging. In The Great Debate, held…

The ACR’s State-of-the-Art Clinical Symposium: Experts Discuss Jakinibs, Osteoarthritis, Membranous Lupus Nephritis
CHICAGO—With the approval of the Jak inhibitors (i.e., jakinibs) tofacitinib and ruxolitinib—and others being investigated—rheumatologists need to arm themselves with an understanding of these drugs so they can think critically when evaluating them and deciding how to use them, said John O’Shea, MD, chief of the Molecular Immunology and Inflammation Branch of and scientific director…

Clinical Rheumatology Year in Review 2021
ACR Convergence 2021—On Nov. 5, Karen H. Costenbader, MD, MPH, professor of medicine, Harvard Medical School, and director of the lupus program, Brigham and Women’s Hospital, Boston, gave a whirlwind review of the most important clinical rheumatology publications of the past year. Testing New Medications for Rheumatic Disease ADVOCATE Trial of Avacopan Dr. Costenbader first…

From Contraception to Breastfeeding: Experts Address Reproductive Health & Medication Management
ACR CONVERGENCE 2021—Rheumatologists are often left in a challenging space when managing medications for patients with rheumatic diseases in relation to contraception, pregnancy and breastfeeding, especially with many novel immunosuppressants and often a dearth of pregnancy safety data. On Nov. 6 during ACR Convergence 2021, leading reproductive health experts came together to speak on this…

Summer 2021’s Awards, Appointments & Announcements in Rheumatology
Marian Hannan Celebrated after 10 Years as AC&R Editor-in-Chief By Kelly April Tyrrell This summer, the 10-year tenure of Marian Hannan, MPH, DSc, as editor in chief of Arthritis Care & Research (AC&R), has come to an end. Kelli Allen, PhD, assumed the post on July 1. “Marian has done a fantastic job over the…
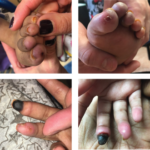
Case Report: A 5-Year-Old with an Ischemic Digit
Janus kinase 1 and 2 inhibitors (jakinibs) have been approved for the treatment of rheumatoid arthritis, psoriatic arthritis and, most recently, juvenile idiopathic arthritis. They have also shown promise in the treatment of interferon (IFN) mediated diseases. The Janus kinase and signal transducer and activator of transcription (JAK/STAT) pathway is the principal signaling pathway for…

FDA Requires New Boxed Warnings on JAK Inhibitors, Places Restrictions on Use
On Sept. 1, the U.S. Food & Drug Administration (FDA) announced that it is requiring revisions to the Boxed Warning for the Janus kinase (JAK) inhibitors Xeljanz/Xeljanz XR (tofacitinib), Olumiant (baricitinib) and Rinvoq (upadacitinib) to include information about the risks of serious heart-related events, cancer, blood clots and death.1 Recommendations for healthcare professionals will include…

20 Years of RA Data: First-Line Biologic & Targeted Synthetic DMARD Trends
Data from a single-center registry shines light on 20 years of trends in first-line, biologic disease-modifying anti-rheumatic drug prescriptions for patients with rheumatoid arthritis.

Janus Kinase Inhibitors Promising for Difficult-to-Treat RA
In a study, Janus kinase inhibitors proved effective for patients with difficult-to-treat rheumatoid arthritis (RA).