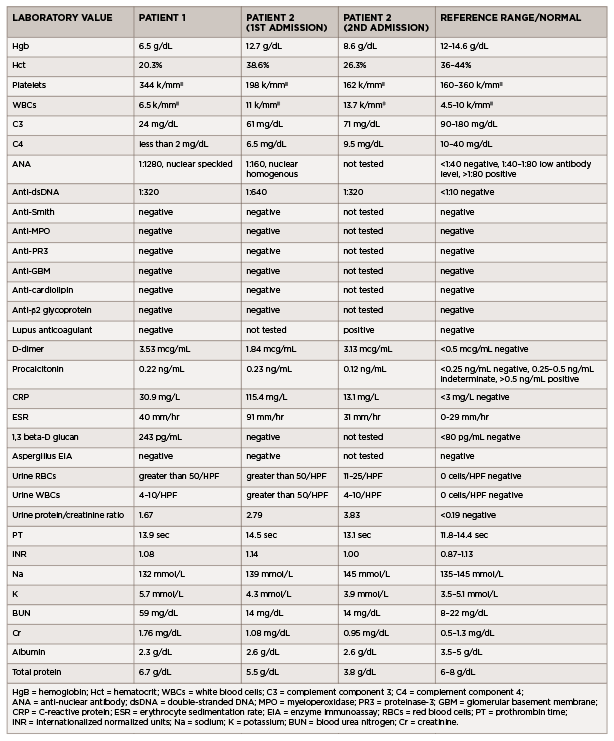
(click for larger image) Table 1: Laboratory Values (all values listed are from the day of admission)
Patient 2
A 35-year-old man with previously well-controlled systemic lupus erythematosus (SLE) with cutaneous features presented with four days of myalgias, generalized fatigue, rhinorrhea, nasal congestion, productive cough with white sputum and subjective fevers, followed by one day of <100 mL of hemoptysis.
He reported no shortness of breath, dyspnea on exertion, nausea, vomiting, hematuria, dysuria, sick contacts or recent travel. His past medical history was noteworthy for having completed latent tuberculosis treatment for a positive, purified-protein-derivative skin test in 2000 and a hospital admission in April 2019 for cough and shortness of breath. During that admission, he was found to have an exudative pleural effusion with numerous neutrophils, histiocytes and multinucleated giant cells. Pulmonary tuberculosis was ruled out, and his illness was attributed to an undefined infection.
The patient was diagnosed in 2008 with lupus with rash, arthritis, hypocomplementemia, thrombocytopenia, positive ANA, positive anti-dsDNA antibody and anti-Smith antibody. His symptoms had been controlled from 2008 to late 2019 with hydroxychloroquine and low-dose prednisone. However, in late 2019, he developed Raynaud’s phenomenon and proteinuria, and was started on mycophenolate mofetil, with limited adherence. At the time of his presentation in early 2021, he had been consistently taking only prednisone and hydroxychloroquine.
In the emergency department, he was afebrile and normotensive, but tachycardic, with a heart rate of 130 beats per minute. His oxygen saturation was 97% on room air, and his COVID-19 nasal swab testing was negative. Initial laboratory testing revealed leukocytosis (see Table 1).
A chest X-ray demonstrated left middle lobe consolidation with a left pleural effusion. Thoracentesis yielded exudative fluid with numerous neutrophils, histiocytes and multinucleated giant cells. Bacterial and fungal cultures from this fluid were negative.
Additional laboratory studies revealed microscopic hematuria, pyuria, proteinuria, hypocomplementemia, positive ANA, positive anti-dsDNA antibody, lupus anticoagulant, elevated C-reactive protein and elevated erythrocyte sedimentation rate. An evaluation for infection, including viral serologies, fungal serum markers, bacterial and fungal blood cultures, and pleural fluid bacterial and fungal cultures, did not reveal an infectious etiology.
A CT of the chest revealed extensive bilateral, perihilar, patchy opacities, patent central airways and areas of bilateral consolidation with groundglass opacities and peripheral sparing.
Bronchoalveolar lavage confirmed the diagnosis of DAH, with increasingly bloody aspirates on serial washings (see Figure 1).
The patient was treated with IV pulse methylprednisolone for three days, with subsequent taper, and one dose of 1 g IV cyclophosphamide. He remained hemodynamically stable on room air throughout his hospital course and never required blood transfusions. He was discharged on oral prednisone daily and hydroxychloroquine, with a plan for monthly IV cyclophosphamide.
One week following discharge, he returned to the emergency department with multiple episodes of hemoptysis and worsening respiratory symptoms. He was tachycardic, with a heart rate of 120 beats per minute and tachypneic, breathing at 22 breaths per minute, with an oxygen saturation of 82% on room air.
His chest X-ray showed slight interval improvement, but a CT pulmonary angiogram demonstrated multiple acute pulmonary emboli, along with extensive multifocal airspace consolidations in both lungs.