In March 2020, an elderly married couple living on Long Island, N.Y., presented to our emergency department with symptoms suspicious for COVID-19 infection.
The wife, a-76-year-old woman, presented with complaints of subjective fevers, minimal dry cough and headaches of one-week duration. She denied having any chills, rhinorrhea, diarrhea, abdominal pain or shortness of breath. Two days earlier, she had presented with similar symptoms, but was discharged home.
Her past medical history was significant for seronegative rheumatoid arthritis, diagnosed in 2006; hypertension; coronary artery disease; hyperlipidemia; and gastroesophageal reflux. She had previously had hand surgery and cataract surgery. She had been on etanercept 50 mg weekly, methotrexate 10 mg weekly for more than 10 years, olmesartan, metoprolol, omeprazole and nortriptyline. She had no recent travel history.
On admission, she had a temperature of 37.6º C, with a pulse of 88 beats/minute, a respiratory rate of 24 breaths/minute and a blood pressure of 184/74 mmHg. Her body mass index (BMI) was 28.1 kg/m2. Her lungs were clear to auscultation and no murmur was appreciated; there were no signs of synovitis or joint deformities; the remainder of her physical exam was reported to be without abnormalities.
Abnormal laboratories included sodium 125 mmol/L (reference range [RR]: 135–146), chloride 83 mmol/L (RR: 96–107), glucose 136 mg/DL (RR: 70–99), alanine transaminase 35 UI/L (RR: 0–33), aspartate transaminase 51 UI/L (RR: 0–32), white blood cell count of 4,550 cells/mm3 (RR: 4,800–10,800), with a lymphocyte count of 250 cells/mm3 (RR: 900–4,800) and a procalcitonin of 0.05 ng/mL (RR: <0.10). Her C-reactive protein (CRP) was 1.2 mg/dL (RR: 0–0.5) on day 3 of hospitalization, and her erythrocyte sedimentation rate (ESR) (RR: 0–30 mm/h) and D-dimer were within normal limits. Her interleukin 6 (IL-6) was 12.8 pg/mL (RR: <14.8). She had a negative QuantiFERON TB test (mitogen 10 IU/mL).
Her chest X-ray and chest computed tomography (CT) were clear, without any infiltrates. Other than an elevated temperature of 38.1° C on hospital day 3, her symptoms quickly abated.
A nasopharyngeal swab obtained on admission was positive for SARS-CoV-2 by polymerase chain reaction (PCR) (Viracor Eurofins, Lee’s Summit, Mo.). She did not require any supplemental oxygen, hydroxychloroquine, azithromycin, nor any antiviral agents; she did receive acetaminophen, but did not receive methotrexate or etanercept during the hospitalization.
Her husband, a 78-year-old man, presented to the emergency department complaining of high fevers (38.9º C) and severe dry cough for the previous 24 hours, along with fatigue, myalgias, shortness of breath, frontal headaches and lightheadedness.
His medical history was significant for ankylosing spondylitis, hypertension, hypothyroidism, benign prostatic hypertrophy, supraventricular tachycardia with ablation, neuropathy and hyperlipidemia. His medications included secukinumab 150 mg subcutaneous every four weeks for the previous 16 months, amlodipine, hydrochlorothiazide, losartan, nortriptyline, levothyroxine, rosuvastatin and tamsulosin.
On admission, the patient’s vital signs were: temperature 38.2° C, respiration rate 22 breaths/minute, blood pressure 179/78 mmHg, pulse oximeter 98% on room air. His weight was 152 kg. On physical exam, he had clear breath sounds on auscultation and no evidence of a murmur. The remainder of his physical exam was within normal limits.
His laboratory values on admission included a sodium level of 133 mmol/L (RR: 135–146), chloride of 95 mmol/L (RR: 96–107), glucose of 107 mg/dL (RR: 70–99), BNP 659 pg/mL (<450 pg/mL). His complete blood count showed thrombocytopenia 141,000/mm3 (RR: 150,000–450,000). His lymphocyte count was normal at 1,150/mm3 (RR: 900–4,800). His CRP was 1.9 mg/dL (RR: 0–0.5).
His chest X-ray on admission was clear and free of infiltrates.
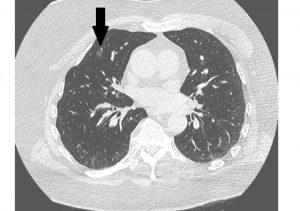
Over the next three days, the patient continued to have fevers and became progressively dyspneic, requiring supplemental oxygen. A chest CT performed on day 2 showed several small areas of groundglass opacities (see Figure 1). On day 3 his lactate dehydrogenase was 611 IU/L, ferritin 843 ng/mL, D-dimer 246 ng/mL and CRP 11.7 mg/dL (all higher than normal ranges). A nasopharyngeal swab obtained on admission was positive for SARS-CoV-2 by PCR.
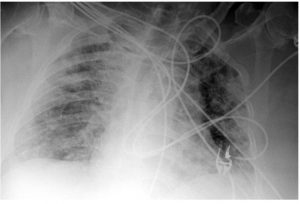
Figure 2. The husband’s day 4 chest X-ray showed interval development of diffuse patchy airspace opacities bilaterally.
On day 4, the patient’s condition worsened. He became more hypoxemic due to interval development of diffuse patchy airspace opacities bilaterally on chest X ray (see Figure 2) requiring intubation and mechanical ventilation. On day 7, the patient remained on mechanical ventilation. He completed a five-day course of hydroxychloroquine, azithromycin and steroids. Secukinumab was not administered during the hospitalization.
Discussion
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is an RNA virus first reported in Wuhan, Hubei Province, China, in December 2019.1 As of April 7, more than 1.2 million people worldwide had been confirmed with COVID19 infection, and more than 72,000 deaths had occurred.2 Mortality is higher in the elderly, but little is known about COVID-19 infection in immunocompromised patients, particularly those with rheumatic diseases on biologics.
We report two cases of a husband and wife, both receiving different disease-modifying anti-rheumatic drugs (DMARDs), with markedly different clinical presentations and hospital courses. The wife, a patient with rheumatoid arthritis who received etanercept and methotrexate prior to hospitalization, had a very mild clinical course that required no major therapeutic intervention. Her husband, with ankylosing spondylitis, had received secukinumab and progressed to critical COVID-19 disease requiring mechanical ventilation.
These cases present some intriguing considerations regarding the immunological underpinning of COVID.
The clinical course of COVID-19 varies from mild to critical, possibly progressing to death. Cytokine storm, macrophage activation syndrome (MAS) and secondary hemophagocytic lymphohistiocytosis (sHLH) are disease processes triggered by infections, autoimmune diseases, drugs or malignancy. Clinically, cytokine storm presents with a syndrome of excessive immune activation that causes fever, pancytopenia, hyperferritinemia, splenomegaly, hepatitis, encephalopathy and coagulopathy. The etiology is thought to be caused by excessive activation and proliferation of T lymphocytes and macrophages with massive hypersecretion of proinflammatory cytokines, including interleukin 1 beta (IL-1β), IL-6, interferon-γ (IFN-γ), and tumor necrosis factor α (TNFα).3,4
Huang et al. described a cytokine profile similar to that in sHLH, which is associated with COVID-19 disease severity. It is characterized by increased IL-2, IL-7, granulocyte-colony stimulating factor, IFN-γ inducible protein 10, monocyte chemoattractant protein 1, macrophage inflammatory protein 1-α and TNFα. It is well known that IL-6 and IL-1β blockade has been successfully used in treating MAS secondary to systemic juvenile idiopathic arthritis (sJIA).5,6 That success has led to clinical trials where more than one-third of patients achieved clinical remission.7,8 Additionally, the IL-1 receptor inhibitor, anakinra, has been associated with improved survival in sepsis with MAS-like features.9 Similarly, the IL-6 antagonist, tocilizumab, could suppress the cytokine storm, as suggested by early promising results in a small cohort of COVID-19 patients.10 Therefore, it is worthwhile exploring whether blocking other cytokines involved in MAS or sHLH may be beneficial in cases of cytokine storm from COVID-19 infection.
TNFα may play a key role in the cytokine storm. TNFα is a 26 kd homotrimeric transmembrane protein expressed on the surface of macrophages, T lymphocytes, natural killer cells, smooth muscle cells and fibroblasts. TNFα, along with IFN-γ, plays a significant role in the immune response against viral infections. TNFα and IFN-γ exhibit a cross-talk at the level of TNF receptor 1 (TNFR1) to induce activation of macrophages. IFN-γ signaling causes nuclear localization of STAT1α that precludes it from being recruited at TNFR1, leading to an enhanced TNFα-induced NFκB activation.11-13
Blocking IFN-γ with emapalumab has been described in successfully treating secondary HLH due to multiple viral infections.14 Emapalumab is a human monoclonal antibody to IFN-γ that acts to block its binding to cell surface receptors and activation of inflammatory signals. Emapalumab is used to treat severe HLH in which serum IFN-γ levels are elevated.15,16
Similarly, TNFα inhibition may theoretically increase the risk of viral infections, but could potentially be beneficial in cases of cytokine storm.17 From the literature on the 2003 SARS-CoV infection, it is suggested the viral infection is associated with decreased angiotensin-converting enzyme 2 (ACE2) expression, leading to an increased activity of the renin-angiotensin system responsible for lung injury.18
The spike protein of SARS-CoV (SARS-S) induces ACE2 shedding via a process tightly linked with TNFα production.19 In an in vitro study, IL-6 and TNFα were up-regulated by the recombinant S protein of SARS-CoV, suggesting that TNF inhibitors and IL-6 antagonists may potentially reduce the inflammatory storm on SARS-CoV-2 and lung damage.20
In our cases, it is possible that pretreatment with etanercept for rheumatoid arthritis in our female patient could have resulted in a blunted IL-6 response indirectly, which may have contributed to her mild case of COVID-19. In support of this hypothesis is the patient’s normal plasma IL-6 level and a minimally elevated CRP, while other inflammatory markers remained within normal limits. Sha et al. demonstrated in a cohort of HIV patients pretreated with etanercept, TNFα and IL-6 levels were blunted after stimulation with IL-2.6 Our case also suggests the possibility that a TNFα inhibitor, such as etanercept, could minimize or abort the potential cytokine storm.
In contrast, COVID-19 infection in the husband was critical. The drug, secukinumab, he was receiving may have aggravated the viral infection. Secukinumab is a human IgG1κ monoclonal antibody that binds to the protein, IL-17A. In host defense, IL-17A has been shown to be mostly beneficial against infection caused by extracellular bacteria and fungi.21 The primary function of T helper (Th) 17 cells appears to be control of the gut microbiota, as well as the clearance of extracellular bacteria and fungi.22,23
IL-17A and IL-17 receptor signaling has been shown to play a protective role in host defenses against many bacterial and fungal pathogens, including Klebsiella pneumoniae, Mycoplasma pneumoniae, Candida albicans, Coccidioides posadasii, Histoplasma capsulatum and Blastomyces dermatitidis.24 However, IL-17A seems detrimental in viral infection, such as influenza, by promoting neutrophilic inflammation.25
It appears the clinical picture of COVID19 is characterized by a strong immune response in the lung parenchyma with mononuclear cells, mainly lymphocytes, more than the direct effect of viral replication.26
An autopsy study of these cases suggested that over-activation of T cells, manifest by an increase of Th17 and high cytotoxicity of CD8 T cells, accounts in part for the severe immune injury. Although the study suggested involvement of Th17, no Th17-associated cytokines were measured in our case. Th17 cells and IL-17 are involved in cytokine storm response. Both IL-1β and TNFα promote Th17 responses, leading to vascular permeability and leakage. Wu et al. proposed that inhibiting Th17 response may be beneficial.27
Conclusion
These two cases highlight the disparate clinical courses that may be experienced by patients with rheumatic disease who are infected by SARS-CoV2. The immunologic mechanisms underpinning the more severe manifestations of COVID-19 have not yet been elucidated, and observations such as these may begin to provide some clarity.
It is important, however, to acknowledge limitations in interpreting these cases. In particular, the clinical course of COVID -19 may be highly heterogeneous, even among patients with otherwise similar characteristics. The relative contribution of other potential risk-factors (e.g., gender, genetic milieu and concomitant medications) is impossible to differentiate from the impact of the biologic agents used for each patient.
That said, these cases raise intriguing questions regarding the pathogenesis of COVID-19. The potential risks and benefits of specific immunosuppressive agents for patients with COVID-19 and the potential role of biologic agents for the management of severe COVID-19 warrant further study.

Dr. Marcos
Luis A. Marcos, MD, MPH, is an infectious diseases physician, Associate Professor, Department of Medicine, Stony Brook University Renaissance School of Medicine, N.Y.

Dr. Sharmeen
Saika Sharmeen, DO, is a rheumatologist and an assistant professor in the Department of Medicine, Division of Rheumatology, Allergy and Immunology, Stony Brook University Renaissance School of Medicine, N.Y.

Dr. Gonzalez
Jaime Gonzalez, MD, is an infectious diseases fellow, Department of Medicine, Stony Brook University Renaissance School of Medicine, N.Y.

Dr. Yao
Qingping Yao, MD, PhD, is a rheumatologist, Professor, Department of Medicine, Chief, Division of Rheumatology, Allergy and Immunology, Stony Brook University Renaissance School of Medicine.

Dr. Fries
Bettina Fries, MD, is an infectious diseases physician, professor and chief of the Division of Infectious Diseases, Department of Medicine, Stony Brook University Renaissance School of Medicine, N.Y.

Dr. Fuhrer
Jack Fuhrer, MD, is an associate professor of medicine and faculty member of the Division of Infectious Diseases. He is also the director of the HIV Treatment Center, and associate dean for admissions for the Renaissance School of Medicine at Stony Brook University, N.Y.
References
- Zhou P, Yang X-L, Wang X-G, et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020 Mar;579(7798):270–273.
- World Health Organization. Coronavirus disease 2019 (COVID-19) Situation Report – 78. 2020 Apr 7. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/situation-reports.
- Kostik MM, Dubko MF, Masalova VV, et al. Identification of the best cutoff points and clinical signs specific for early recognition of macrophage activation syndrome in active systemic juvenile idiopathic arthritis. Semin Arthritis Rheum. 2015 Feb;44(4):417–422.
- Parodi A, Davì S, Pringe AB, et al. Macrophage activation syndrome in juvenile systemic lupus erythematosus: A multinational multicenter study of thirty-eight patients. Arthritis Rheum. 2009 Nov;60(11):3388–3399.
- Huang C, Wang, Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020 Feb 15;395(10223):497–506.
- Grom AA, Horne A, De Benedetti F. Macrophage activation syndrome in the era of biologic therapy. Nat Rev Rheumatol. 2016 May;12(5):259–268.
- Ruperto N, Brunner HI, Quartier P, et al. Two randomized trials of canakinumab in systemic juvenile idiopathic arthritis. N Engl J Med. 2012 Dec 20;367(25):2396–2406.
- De Benedetti F, Brunner HI, Ruperto N, et al. Randomized trial of tocilizumab in systemic juvenile idiopathic arthritis. N Engl J Med. 2012 Dec 20;367(25):2385–2395.
- Shakoory B, Carcillo JA, Chatham WW, et al. Interleukin-1 receptor blockade is associated with reduced mortality in sepsis patients with features of macrophage activation syndrome: Reanalysis of a prior phase III trial. Crit Care Med. 2016 Feb;44(2):275–281.
- Xu X, Han M, Li T, et al. Effective treatment of severe COVID-19 patients with tocilizumab. ChinaXiv:20200300026. 2020.
- Wesemann DR, Benveniste EN. STAT-1 alpha and IFN-gamma as modulators of TNF-alpha signaling in macrophages: regulation and functional implications of the TNF receptor 1:STAT-1 alpha complex. J Immunol. 2003 Nov 15;171(10):5313–5319.
- Parameswaran N, Patial S. Tumor necrosis factor-α signaling in macrophages. Crit Rev Eukaryot Gene Expr. 2010;20(2):87–103.
- Paludan SR. Synergistic action of pro-inflammatory agents: Cellular and molecular aspects. J Leukoc Biol. 2000 Jan;67(1):18–25.
- Lounder DT, Bin Q, de Min C, Jordan MB. Treatment of refractory hemophagocytic lymphohistiocytosis with emapalumab despite severe concurrent infections. Blood Adv. 2019 Jan 8;3(1):47–50.
- Vallurupalli M, Berliner N. Emapalumab for the treatment of relapsed/refractory hemophagocytic lymphohistiocytosis. Blood. 2019 Nov 21;134(21):1783–1786.
- Wesemann DR, Benveniste EN. STAT-1 alpha and IFN-gamma as modulators of TNF-alpha signaling in macrophages: Regulation and functional implications of the TNF receptor 1:STAT-1 alpha complex. J Immunol. 2003 Nov 15;171(10):5313–5319.
- Ali T, Kaitha S, Mahmood S, et al., Clinical use of anti-TNF therapy and increased risk of infections. Drug Healthc Patient Saf. 2013;5:79–99.
- Zou Z, Yan Y, Shu Y, et al. Angiotensin-converting enzyme 2 protects from lethal avian influenza A H5N1 infections. Nat Commun. 2014 May 6;5:3594.
- Haga S, Yamamoto N, Nakai-Murakami C, et al. Modulation of TNF-alpha-converting enzyme by the spike protein of SARS-CoV and ACE2 induces TNF-alpha production and facilitates viral entry. Proc Natl Acad Sci U S A. 2008 Jun 3;105(22):7809–7814.
- Wang W, Ye L, Ye L, et al. Up-regulation of IL-6 and TNF-alpha induced by SARS-coronavirus spike protein in murine macrophages via NF-kappaB pathway. Virus Res. 2007 Sep;128(1–2):1–8.
- Kolls JK, Khader SA. The role of Th17 cytokines in primary mucosal immunity. Cytokine Growth Factor Rev. 2010 Dec;21(6):443–448.
- Kumar P, Monin L, Castillo P, et al. Intestinal interleukin-17 receptor signaling mediates reciprocal control of the gut microbiota and autoimmune inflammation. Immunity. 2016 Mar 15;44(3):659–671.
- Ivanov II, Atarashi K, Manel N, et al. Induction of intestinal Th17 cells by segmented filamentous bacteria. Cell. 2009 Oct 30;139(3):485–498.
- Chen K, Kolls JK. T cell-mediated host immune defenses in the lung. Annu Rev Immunol. 2013;31:605–633.
- Crowe CR, Chen K, Pociask DA, et al. Critical role of IL-17RA in immunopathology of influenza infection. J Immunol. 2009 Oct 15;183(8):5301–5310.
- Xu Z, Shi L, Wang Y, et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020 Apr;8(4):420–422.
- Wu D, Yang XO. TH17 responses in cytokine storm of COVID-19: An emerging target of JAK2 inhibitor fedratinib. J Microbiol Immunol Infect. 2020 Mar 11 [online ahead of print].
Updated and revised April 13, 2020.