SAN DIEGO—In recognition of the growing interest in and importance of the preclinical phases of rheumatic diseases, a panel of experts convened at the 2013 ACR/ARHP Annual Meeting, held October 26–30, to discuss key issues to better understand the etiology and early pathology of rheumatic diseases. This article summarizes data from select presentations given at this conference, including current investigation into the mucosal biology in the etiology of rheumatic diseases and ways to detect early organ injury in preclinical rheumatic diseases. [Editor’s Note: These sessions were recorded and are available via ACR SessionSelect at www.rheumatology.org.]
Mucosal Biology in the Etiology of Rheumatic Diseases
Jose U. Scher, MD, assistant professor of medicine and director of the Microbiome Center for Rheumatology and Autoimmunity at NYU-Hospital for Joint Diseases in New York, and Anca I. Catrina, MD, PhD, in the department of rheumatology at the Karolinska University Hospital, Karolinska Institute, in Stockholm, provided an update on the long-discussed potential role of the human microbiome in the pathogenesis of rheumatoid arthritis (RA).
In his talk, “Microbiome and Initiation and Propagation of Rheumatoid Arthritis,” Dr. Scher emphasized that emerging data are implicating the human microbiome in the pathogenesis of RA, as evidenced by studies that find an association of RA with various mucosal sites. “Mucosal sites exposed to a high load of bacterial antigens, such as the periodontium, lung, and gut, may represent the initial site of autoimmune generation or even the ‘second hit’ leading to emergence of RA clinical manifestations,” he said.
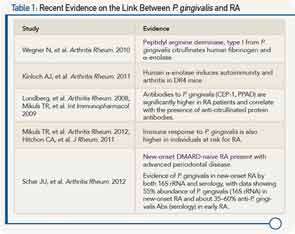
Currently, evidence of this association is strongest between oral microbiota and RA, and Dr. Scher walked participants through a number of more recent studies that he said are advancing the evidence on the link between Porphyromonas gingivalis (P. gingivalis) and RA (see Table 1).
He also presented new data suggesting an association between gut dysbiosis and RA, highlighting key findings from a recently published study that found a high abundance of Prevotella in new-onset RA (NORA) patients, and an increased sensitivity to dextran sulfate sodium (DSS) colitis associated with the presence of Prevotella.1 Further evidence on an association between Prevotella and new-onset RA, he said, is a study that showed that humans can be clustered into three groups, or enterotypes, according to gut microbiota, of which Prevotella is one enterotype. The study showed that although only 13% of people belong to the Prevotella enterotype, 75% of new-onset RA belong to that enterotype.
The association of RA and the lungs was discussed by Dr. Catrina during her talk, “Lung Inflammation and the Development of RA-Related Autoimmunity.” She presented data from studies showing an association between the presence of specific anti-citrullinated protein antibodies (ACPA) and parenchymal lung abnormalities detected by high-resolution computer tomography, and the presence of local citrullination and anti-citrulline immunity in the lungs early in the development of ACPA-positive RA (see “Key References on Evidence for Lung Inflammation and RA,”).
According to Dr. Catrina, these results suggest that early inflammatory lung events may play a critical role in initiating the development of ACPA-positive RA. “Further studies to address the complex relationship between inflammation in the lungs and in the joints are warranted,” she said. “Such studies will allow identification of environmental factors and early immune events that can be targeted with preventive or therapeutic measures.”
Dr. Scher also emphasized the importance of future studies to validate the current evidence emerging on an association between these mucosal environments and the development of RA by proving causation. “If validated, these findings could lead to the discovery of potential biomarkers and therapeutic approaches at the preclinical and clinical phases of RA,” he said.
Detecting Early Organ Injury in Preclinical Rheumatic Diseases
In a session titled, “Symptom Assessment and Joint Examination in Pre-Clinical Rheumatoid Arthritis: How To Assess in an Accurate, Valid, and Reproducible Fashion?” Vivian P. Bykerk MD, director of the Inflammatory Arthritis Center of Excellence at the Hospital for Special Surgery in New York City, provided information on how to accurately assess symptoms and signs in people at risk for developing inflammatory/rheumatoid arthritis (IA/RA) and how to determine a person’s risk for developing RA based on the presence of certain signs and symptoms.
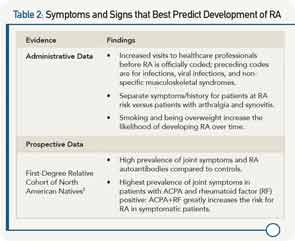
Using information and criteria from recently published recommendations for terminology and research in individuals at risk of RA by the European League Against Rheumatism that define specific phases of RA, she focused her talk on the phase of disease in which patients have symptoms without clinical evidence of arthritis.2 To help clinicians assess these persons at risk for developing RA, she reviewed the current evidence on what symptoms and signs best predict who will develop overt persistent synovitis or classifiable RA (see Table 2).
She also highlighted recent data from imaging studies that show the detection of RA in ACPA-positive patients prior RA diagnosis. A study by Krabben and colleagues conducted to assess whether local inflammation occurs in the preclinical phase of RA found that subclinical inflammation was detected by magnetic resonance imaging in ACPA-positive patients with arthralgia prior to synovitis or even symptoms of RA.4 In another study that examined the relationship between the presence of ACPA and osteoclast differentiation in people with RA-related autoimmunity, Kleyer and colleagues found cortical bone changes (e.g., thinning, fenestration, and small bone erosions) in asymptomatic ACPA-positive people.5
Currently, there are no data or recommendations on how often symptomatic patients should be followed, she said, but emphasized the importance given data that show that recurrent symptoms contribute to the risk of developing RA. To help clinicians predict which patients with arthralgia will develop RA, she referred them to a prediction tool developed from the Amsterdam Arthralgia Cohort.6 The tool uses nine variables that are scored (range, 0–13) to predict RA risk. The nine variables include RA in a first-degree family member, alcohol non-use, duration of symptoms less than 12 months, presence of intermittent symptoms, arthralgia in upper and lower extremities, visual analogue scale pain ≥50, presence of morning stiffness ≥1 hour, history of swollen joints as reported by the patient, and antibody status. Patients are then categorized into three risk groups: low (0–4 points), intermediate (5–6 points), and high risk (7–13 points).
Overall, she emphasized the need for studies to evaluate prevention in patients at risk of developing RA. “In general, a combination of symptoms and autoantibodies yields the highest risk for RA and may be the most feasible population to target for a prevention intervention,” she said.
Mary Beth Nierengarten is a freelance medical journalist based in St. Paul, Minn.
Key References on Evidence for Lung Inflammation and RA
- Klareskog L, Catrina AL, Paget S. Rheumatoid arthritis. Lancet. 2009;373 659-672.
- Demoruelle MK, Weisman MH, Simonian PL, Lynch DA, Sachs PB, Pedraza IF, et al. Brief report: Airways abnormalities and rheumatoid arthritis-related autoantibodies in subjects without arthritis: Early injury or initiating site of autoimmunity. Arthritis Rheum. 2012; 64:1756-1761.
- Reynisdottir G, Karimi R, Joshua V, Olsen H, Hensvold AH, Harju, A, et al. Structural lung changes and local anti-citrulline immunity are early features of anti-citrullinated-proteins antibodies positive rheumatoid arthritis. Arthritis Rheum. 2013 Oct 21. doi: 10.1002/art.38201. [Epub ahead of print]
References
- Scher JU, Sczesnak A, Longman RS, Segata N, Ubeda C, Bielski C. Expansion of intestinal Prevotella copri correlates with enhanced susceptibility to arthritis. Elife. 2013;2.pii:e01202.
- Raza K, Saber TP, Kvein TK, Tak PP, Gerlag DM. Timing the therapeutic window of opportunity in early rheumatoid arthritis: Proposal for definitions of disease duration in clinical trails. Ann Rheum Dis. 2012;71:1921-1923.
- Smolik I, Robinson DB, Bernstein CN, El-Gabalawy HS. First-degree relatives of patients with rheumatoid arthritis exhibit high prevalence of joint symptoms. J Rheumatol. 2013;40:818-824.
- Krabben A, Stomp W, van der Heijde DM, van Nies JA, Bloem JL, Huizinga TW, et al. MRI of hand and foot joints of patients with anticitrullinated peptide antibody positive arthralgia without clinical arthritis. Ann Rheum Dis. 2013;72:1540-1544.
- Kleyer A, Finzel S, Rech J, Manger B, Krieter M, Faustini F, et al. Bone loss before the clinical onset of rheumatoid arthritis in subjects with anticitrullinated protein antibodies. Ann Rheum Dis. 2013 Mar 21 [Epub ahead of print].
- van de Stadt LA, Witte BI, Bos WH, van Schaardenburg D. A prediction rule for the development of arthritis in seropositive arthralgia patients. Ann Rheum Dis. 2013; 72:1920-1926.