What is freedom? It is first, the capacity to deliberate or to weigh alternatives.
—Martin Luther King, Jr.
SAN DIEGO—Across from the San Diego Convention center, running along a path from Market Street to PETCO Park next to the trolley line, is the Martin Luther King, Jr. promenade, a walkway interspersed with concrete slabs etched with sayings by Dr. King. I happened to walk by the concrete slab with the saying shown in the above epigram shortly after sitting in a session billed as, “The Great Debate: Biologics or Triple Therapy for the Treatment of Rheumatoid Arthritis,” here at the 2013 ACR/ARHP Annual Meeting, held October 26–30. [Editor’s Note: This session was recorded and is available via ACR SessionSelect at www.rheumatology.org.] During the session, the respective merits of biologics and triple therapy for the treatment of patients with rheumatoid arthritis (RA) were debated. James O’Dell, MD, and Ronald van Vollenhoven, MD, certainly fulfilled the billing as lively debaters, but in the end, the real news for the practicing rheumatologist is that they now have more good alternatives to weigh when deliberating on which treatment strategy works best for their patients.
When asked who he thought “won” the debate, session moderator Eric Matteson, MD, chair of rheumatology and professor of medicine at the Mayo Clinic in Rochester, Minn., said, “I think that the big winners are our rheumatologists and patients, who now have carefully considered options for effective treatment.”
“In my view,” he continued, “the field is set to move to the next stage—namely, to determine from disease onset, and also at point of determination of the need to switch therapy, which therapy is most appropriate for which patient from a safety, efficacy, and cost standpoint.”
The debate, he said, highlighted for rheumatologists the practical issues they and their patients face daily and offered an insightful appraisal of the best current knowledge and clinical science for triple therapy and biologics.
Triple Therapy for RA
James R. O’Dell, MD, professor of internal medicine in the division of rheumatology at Nebraska Medical Center in Omaha, opened the debate by framing his advocacy of triple therapy for RA over biologics through two general, interrelated themes: 1) The importance of managing patients with a treat-to-target approach; and 2) The need to consider the high cost of biologics. Overall, he said that many patients will not need biologics if managed with a treat-to-target approach that incorporates the use of triple therapy for patients who need more aggressive treatment.
“All patients should be treated to get their disease to at least a low level of disease activity (even better, to remission),” he said. He emphasized that rheumatologists need to be more aggressive in their use of methotrexate (MTX) in this stepped-up approach to disease management with the conventional disease-modifying antirheumatic drugs (DMARDs).
He urges rheumatologists to start with aggressive treatment with MTX that may include pushing the dose up to 25 mg per week subcutaneously. “This will control 28% to 45% of patients,” he said. In patients in whom this is an insufficient target to control active disease, he advises using triple therapy. “This will control 25% more of patients,” he said. If this treatment still doesn’t reach the target, he then would move on to the use of biologics.
He emphasized, however, that before patients move on to expensive biologics they should use triple therapy.
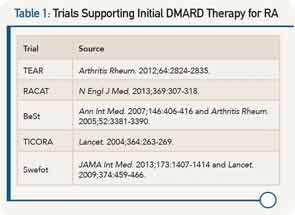
To support this treat-to-target approach, he cited evidence from five investigator-initiated trials over the past 10 years that he said show that conventional DMARD therapy should be the first option in the initial treatment of RA and in patients with active disease despite treatment with MTX (see Table 1).
He said that the TEAR, BeSt, and TICORA trials all clearly show that patients with new-onset RA treated to target with strategies that start with DMARD combinations have comparable clinical outcomes to patients started on biologics. He also highlighted the most recent findings from the RACAT trial that showed for the first time that triple therapy is noninferior to etanercept clinically and radiographically in patients with RA who have active disease despite MTX treatment.
In discussing the Swefot trial, he pointed out that this was not a treat-to-target trial and emphasized that the data from the trial showed no clinical differences at six, nine, or 24 months between patients with early RA who fail MTX treated with the addition of infliximab or those treated with conventional DMARDs.
Underlying the focus on the treat-to-target approach was the emphasis on trying the lower-cost DMARDs before moving on to the much costlier biologics. He used the analogy of a three-legged stool to emphasize the three main issues that need to be considered to support choosing one therapy over another. Along with efficacy and toxicity, cost is equally important to consider. “No healthcare system in the world can afford to have more than 50% of patients with RA on biological therapy,” he said, adding that, fortunately, with the data now available, quality care can be provided much less expensively.
Biologic Therapy for RA
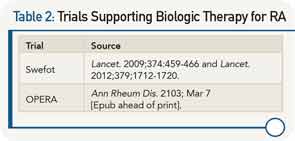
Ronald van Vollenhoven, MD, PhD, professor in the unit for clinical therapy research, inflammatory diseases, at the Karolinska Institute in Stockholm, Sweden, rested his argument on the superior efficacy of biologics as demonstrated in a number of clinical trials (see Table 2). He walked through the data from the one- and two-year results of the Swefot trial, emphasizing that the lack of significantly superior clinical results with the addition of infliximab to MTX compared to the addition of conventional DMARDs to MTX seen at two years could be due to the smaller sample size over time.
He highlighted data from the OPERA trial that showed that adding a biologic to intensive conventional therapy provided even better outcomes, and that results from the RACAT trial show that conventional therapies can achieve decent responses, but that biologics remain unsurpassed in making some patients do really well and preventing structural damage, as noted by the better radiographic findings with the biologics.
Although he focused more heavily on the efficacy data as the most important evidence on which to base treatment decisions, he did acknowledge the high cost of biologics that prohibit their use in large patient groups. He emphasized the need for a smarter use of biologics, which includes their use in induction-maintenance strategies (i.e., starting patients on a biologic to control disease, but then using conventional therapies for maintenance therapy) and adjusting their dose (i.e., studies indicate that in some patients good results can be achieved with half-dose as opposed to full-dose etanercept).
He also emphasized the need for better personalized approaches to treating patients with RA. “This has been the ‘holy grail’ of therapeutics, to predict at the individual-patient level which drug is the right one for them,” he said. Although he said work is still needed in this area, he mentioned research by his group that suggests the utility of a multibiomarker disease-activity test to identify patients who are and who are not at risk for radiographic joint damage. If the test is confirmed, he said, it could help in choosing the optimal therapy for a given patient.
Overall, Dr. van Vollenhoven said he and Dr. O’Dell agreed more than perhaps the debate format permitted. “Most rheumatologists understand these issues and are dealing with them the best they can,” he said, adding that he hopes he provided some key information that will help clinicians provide the best treatment for each patient.
Mary Beth Nierengarten is a freelance medical journalist based in St. Paul, Minn.