“After a change in homeostasis in the periphery, say after an infection, lymphopenia, inflammation or even aging,” he said, “the regulatory T cells in this environment are in a difficult position because of, in the case of CD4 Treg, an excess of inflammatory cytokines and less access to IL-2. Without the interception of Helios, these cells may die or become effector cells.”
The effect of Helios on CD8 Treg is similar. “Without the interception of Helios, these cells may undergo apoptosis,” he said.
In both situations, he said, these effects of Helios would result in enhanced levels of autoimmune tissue destruction.
Looking Ahead
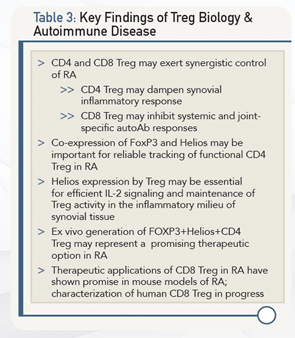
According to Dr. Cantor, further insight into both CD4 Treg and CD8 Treg, particularly as they relate to Helios, will pay off in terms of future ways to diagnose and treat RA. Table 3 lists key findings from current research that is leading toward this goal, and Table 4 lists key studies.
Although currently in its infancy, Treg-based therapy has the potential to help patients with RA and other autoimmune diseases. Critical to achieving this, Dr. Cantor said, is finding a way to expand Helios+ FoxP3+ regulatory T cells, cells that are potent suppressors of autoreactive CD4 T cells.
Mary Beth Nierengarten is a freelance medical journalist based in St. Paul, Minn.
Second Chance
If you missed this session, it’s not too late. Catch it on SessionSelect.