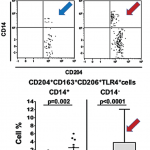
How macrophages (shown) behave when recognizing damage-associated molecular pathways can tell us why some inflammation doesn’t resolve, but becomes chronic and destructive.
royaltystockphoto.com/shutterstock.com
SAN FRANCISCO—To unravel how out-of-control inflammation begins in rheumatoid arthritis and other diseases, one target for immunologists is the macrophage. Researchers discussed macrophage activation and other key drivers of inflammation at the 2015 ACR/ARHP Annual Meeting on Nov. 7. How macrophages behave when recognizing damage-associated molecular pathways (DAMPs) tells us more about why some inflammation doesn’t resolve as it should, but instead becomes chronic and destructive.
Macrophage Polarization
Macrophages are involved in both adaptive and innate immunity. There appears to be some interaction or cross-talk between various cells that affect macrophage polarization, said Lionel B. Ivashkiv, MD, chief scientific officer of the Hospital for Special Surgery in New York.
Stromal cells, including fibroblast-like synoviocytes, may regulate how macrophages respond to tumor necrosis factor, as well as modulate synovial macrophage phenotypes that play a key role in rheumatoid arthritis pathogenesis.1
“Macrophages and fibroblast-like synoviocytes are closely juxtaposed in RA-inflamed synovium,” said Dr. Ivashkiv. These two types of cells would normally work together in balance to keep the joint and synovial fluid in a healthy state, but in RA, something goes wrong, and they lead to chronic inflammation and joint damage.
In the past, macrophage polarization was viewed as taking one of two roads, based on what type of cytokine, such as tumor necrosis alpha or interferon gamma, the cells encountered. The two models were M1 or classical, which would trigger an inflammatory response, and M2 or alternative, which would normally lead to resolution of inflammation and tissue healing.2 In chronic inflammatory diseases, resolution doesn’t happen. RA synovial macrophages have more of a complex polarization spectrum, one where there may be a lot of overlap in gene expression, he said.3
Fibroblast-like synoviocytes modulate the expression of TNF-inducible genes in macrophages, he said. They also superinduce growth factor and prostaglandin pathways in macrophages.4
Synovial macrophages in RA are similar to TPP or C9 macrophages, which are also associated with chronic inflammation. “There’s also a striking similarity between the C9 polarization state and synovial fibroblast-trained macrophages,” he said. “What underlies this synergy?”
Fibroblasts and TNF cooperate to activate transcription factors in macrophages in a PG-dependent manner, and TNF and PGE2 also synergistically induce gene expression.5 Prostaglandins and growth factors also play a role in macrophage polarization. Synovial fibroblast-derived PGEs modulate macrophage TNF responses.