
The effects of pollution (such as that shown here in Beijing, China) do not appear until later in the RA disease process.
Image Credit: designbydx/shutterstock.com
SAN FRANCISCO—Evolving research into the pathogenesis of rheumatoid arthritis (RA) is increasingly showing that rather than a single causative dysfunctional pathway leading to disease, multiple pathways are involved, the study of which can shed additional light on what is occurring in a person’s body prior to developing symptoms of disease.
Saying it another way, no longer are researchers looking for an “original sin” (or autoimmune response to a singular peptide) that causes RA; rather, this immunological concept is being replaced by the metaphor of a “gathering storm” of multiple autoantigen reactions that may precede the onset of clinical disease, according to V. Michael Holers, MD, Scoville Professor and head, Division of Rheumatology, University of Colorado School of Medicine, Aurora, Colo. Such research is promising to reveal new potential targets for treatment, and importantly, ways to potentially prevent the disease from developing at all.
“In people who will ultimately get rheumatoid arthritis, certain autoantibodies and likely autoimmune T cells are already present, and in a poorly understood process, they start to damage joints,” said Dr. Holers during the Rheumatology Research Foundation Memorial Lecture honoring L. Emmerson Ward, MD, at the 2015 ACR/ARHP Annual Meeting. In his presentation, Rheumatoid Arthritis Mechanisms and Predictors of Pre-Clinical RA, Dr. Holers provided an overview of several advances made since the time of Dr. Ward in the understanding of how RA develops.

Dr. Holers
Among these advances is a better understanding of the multiple pathophysiological processes that are occurring in a person before any symptoms of the disease become manifest. According to Eric Matteson, MD, MPH, chair, Rheumatology Department, Mayo Clinic, Rochester, Minn., who moderated the session, one of the most important advances Dr. Holers talked about is the insight that abnormal humoral autoimmunity and related B cell and plasma cell function, along with T cell dysregulation, are involved in the pathway to developing clinical RA.
“These processes begin long before the arthritis becomes apparent, and may take place, for example, in the lung,” Dr. Matteson said. “In some patients, these responses show up as rheumatoid factor or cyclic citrullinated peptide antibodies even before diagnosis.”
(click for larger image)
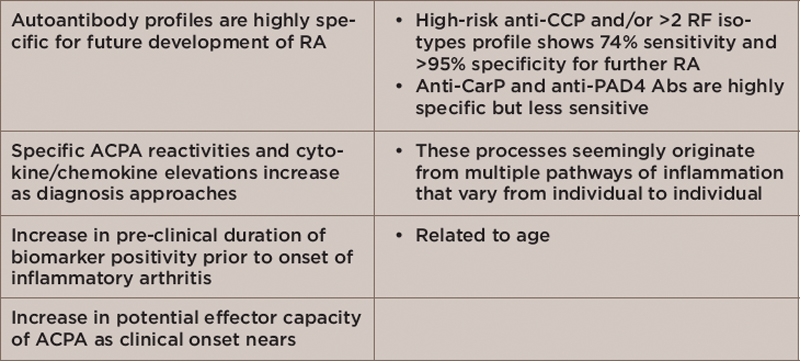
Table 1: Pathophysiological Changes in Persons with Preclinical Disease or Those at High Risk of Developing RA
Key: RA, rheumatoid arthritis; CCP, cyclic citrullinated peptide; RF, rheumatoid factor; CarP, carbamylated protein; PAD4 Abs, peptidylarginine deiminase 4 antibodies; ACPA, anti-citrullinated protein antibodies
In his talk, Dr. Holers described ongoing research examining the pathologic process at RA initiation—that is, what is happening in the body prior to a patient exhibiting synovitis. Table 1 highlights key findings from military cohort studies that show which patients have preclinical disease or are at high risk of developing RA.1,2
New Understanding of How RA Begins: From Bad Luck to Causal Pathways

Dr. Matteson
Saying that most clinicians traditionally preferred to view the initiation of RA as due to bad luck or some uncertain process (stochastic model of disease), Dr. Holers said that the weight of the evidence is now leaning toward a deterministic model in which the disease is thought to be caused by specific and definable environmental and genetic influences.
Results from epidemiological studies of persons at high-risk of developing RA or those with preclinical disease support this, he said. Table 2 lists a number of findings from these studies.3-7
Of particular interest, he said, is evidence showing that omega-3 fatty acids may be protective against the development of RA. He described data showing that an increasing percentage of omega-3 fatty acids in red blood cells is inversely related to anti-CCP2 and lower odds of RF positivity in shared epitope positive individuals who have not yet developed clinically apparent disease.8,9

(click for larger image)
Table 2: Results from Epidemiological Studies of Factors Associated with RA
Key: RA, rheumatoid arthritis; RF, rheumatoid factor; ACPA, anti-citrullinated protein antibody
Dr. Holers emphasized that these epidemiological findings suggest that smoking, contraceptive use and omega-3 fatty acids have an effect on pre-clinical autoimmunity. Unlike these factors, the effects of pollution and development of RA comorbidities do not appear until later in the disease process when initial signs and symptoms of RA appear.
Lung: Primary Site of Disease Initiation?
Emerging from these studies on the multiple changes occurring in pre-clinical disease is an evolving understanding of the involvement of the lungs in RA. Although largely seen as a secondary outcome associated with joint disease, evidence now suggests that the lungs may be affected before the joints and be a primary site of disease initiation.
“The lungs are an area of increasing focus for RA pathogenesis research,” said Dr. Holers. “Think about how big the lung’s surface area is. If you stretch out all the tissue, it’s like a football field. The lungs have so much exposure to various factors in the environment, like viruses and bacteria.”
Evidence is now strongly pointing to the lung as a primary site of origin of RA, said Dr. Holers, prompting a new experimental focus on a potential mucosal-initiation etiology in the pathogenesis of early RA. Such evidence includes data showing an association between persons at risk of or with early RA and environmental exposures (e.g., to smoking, silica, airborne particulate matter), the presence of immunoglobulin A (IgA) autoantibodies, pulmonary abnormalities on high-resolution computed tomography (HRCT), local production of ACPA in the lung, expansion of IgA producing plasmablasts in at-risk individuals, periodonitits and mucosal dysbiosis.
Still not fully understood, he said, is the transition from this pre-clinical early stage of disease to synovitis of which environmental and genetic factors could play a role. A major question needing an answer is at what specific point do environmental factors, such as smoking exposure or omega-3 fatty acids intake, play a role in disease development. “We need prospective, natural history studies to understand how this entire process occurs,” he said, along with better knowledge of how genetic factors influence this process.
Moving Toward Prevention
Along with providing better targeted therapeutics, underlying this research is the primary aim of preventing RA. “The field is moving toward prevention,” said Dr. Holers, adding that in the future rheumatologists may be able to detect patients at risk of RA or with the very earliest warning signs of disease by, for example, sputum testing that may reveal early pathophysiologic changes as described above.
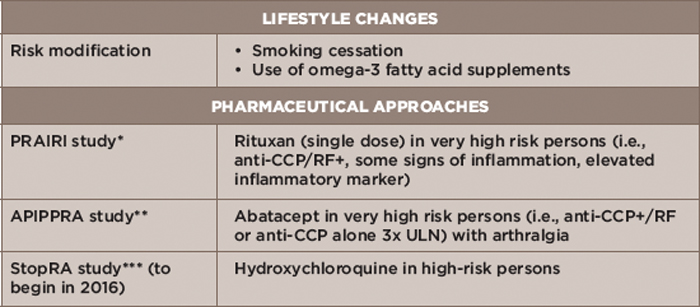
Table 3: Prevention Strategies
Key: CCP, cyclic citrullinated peptide; RF, rheumatoid factor; ULN, upper limit of normal
Notes: *Prevention of Clinically Manifest Rheumatoid Arthritis by B Cell Directed Therapy in the Earliest Phase of the Disease
**Arthritis Prevention in the Pre-Clinical Phase of RA with Abatacept
***Strategy to Prevent the Onset of Clinically Apparent Rheumatoid Arthritis
To that end, a number of RA prevention approaches are under investigation (see Table 3).
Also under consideration is general population screening for either single or multiple diseases based on the rationale of the prevalence of autoimmune disease (about 8% of the population) and the highly specific and unique autoantibodies that can be detected prior to the clinical onset of autoimmune disease.
Epidemiological findings suggest that smoking, contraceptive use & omega-3 fatty acids have an effect on pre-clinical autoimmunity. Unlike these factors, the effects of pollution & development of RA comorbidities do not appear until later in the disease process when initial signs & symptoms of RA appear.
According to Dr. Holers, one strategy for implementing screening would be to first test it in a pilot population (e.g., families with children or people with high-risk genotypes). He also said that an effort to accelerate development of array-based screening across immune diseases is ongoing within studies funded by the National Institutes of Health.
“I expect that the next generation of rheumatologists will experience dramatic changes in the way that RA is evaluated and treated, with an increasing focus on screening and prevention and the identification of novel therapeutic targets and strategies in the preclinical period,” said Dr. Holers. “Consider how cardiovascular disease prevention has evolved over the last 30 years, and expect the same in RA and other rheumatologic and autoimmune diseases.”
Mary Beth Nierengarten is a freelance medical journalist based in St. Paul, Minn.
Second Chance
If you missed this session, it’s not too late. Catch it on SessionSelect.
References
- Sokolove J, Bromber R, Deane KD, et al. Autoantibody epitope spreading in the pre-clinical phase predicts progression. PLoS ONE. 2012;7(5):335296.
- Gan RW, Trouw LA, Shi J, et al. Anti-carbamylated protein antibodies are present prior to rheumatoid arthritis and are associated with its future diagnosis. J Rheumatol. 2015;42(12).
- Kolfenbach JR, Deane KD, Derber LA, et al. A prospective approach to investigating the natural history of preclinical rheumatoid arthritis using first-degree relatives of probands with RA. Arthritis Rheum. 2009;61(12):1735–1742.
- Bhatia SS, Majka DS, Kittelson JM, et al. Rheumatoid factor seropositivity is inversely associated with oral contraceptive use in women without rheumatoid arthritis. Ann Rheum Dis. 2007;66(2):267–269.
- Feser M, Derber LA, Deane KD, et al. Plasma 25, OH vitamin D levels are not associated with rheumatoid arthritis-related autoantibodies in individuals at elevated risk for rheumatoid arthritis. J Rheumatol. 2009;36(5):943–946.
- Gan RW, Deane KD, Zerbe GO, et al. Relationship between air pollution and positivity of ra-related autoantibodies in individuals without established RA: A report on SERA. Ann Rheum Dis. 2013;72(12).
- Norris JM, Gan RW, Hughes-Austin JM, et al. Lack of association between preclinical markers for cardiovascular disease and rheumatoid arthritis-related autoimmunity in first-degree relatives without rheumatoid arthritis. American College of Rheumatology 2013 Meeting Abstract #116.
- Gan RW, Young KA, Zerbe GO, et al. Lower omega-3 fatty acids are associated with the presence of anti-cycliccitrullinated peptide autoantibodies in a population at risk for future rheumatoid arthritis: A nested case-control study. rheumatology 2015 Sep 13. pii: kev266. [Epub ahead of print].
- Gan RW, Demoruelle MK, Deane KD, et al. Omega-3 fatty acids are associated with a lower prevalence of autoantibody positivity in HLA-DR shared epitope positive subjects who are at increased risk for future RA. American College of Rheumatology 2013 Meeting Abstract #2021


