Prior to developing the list of recommendations, the group drafted a set of overarching principles on the management of PsA that were agreed upon by a near consensus (≥80%) (see Table 1).1,3
Using a modified Grading of Recommendations, Assessment, Development and Evaluation (GRADE) process, the panel then developed treatment recommendations for each disease domain (see Table 2) (as well as comorbidities) and a schema that incorporated the overarching principles.1
Dr. Kavanaugh emphasized that these treatment recommendations are meant to help clinicians optimize their care for patients with PsA. The recommendations do not, however, provide clear guidance on exactly when to use each agent.
“I think that when recommendations come out there is a hope or aspiration that they may be more specific than they can be,” he says, “meaning that along with listing the recommended therapies, the specifics on when to use which drug first in a given patient [would] also [be] provided.”
“That would be nice, but we don’t have that information, so the clinician is left with clinical experience and patient choice,” he adds. “So that is partially why more patient involvement is included in these guidelines compared to older guidelines.”
Dr. Deodhar emphasizes that clinicians will want to know how to use these new drugs, whether as first-line treatment or as second-line treatment after anti-TNF inhibition failure. Given the lack of head-to-head studies on comparative efficacy of the drugs, he says that clinicians can look at their comparative safety profiles.
For example, when comparing the safety of methotrexate to the new small-molecule drug, apremilast, he says apremilast seems to be extraordinarily safe except for stomach upset and diarrhea and appears not to be associated with the more severe side effects of liver function abnormalities or significant immunosuppression.
“However, we have 40 years of experience with methotrexate and only three years of experience with apremilast, so the side effects may appear later,” he cautions.
Of course, cost is also an issue, because the cost of the newer agents is extraordinary, he says.
The new recommendations include three new classes of drugs that have come to the market since the 2009 recommendations. These include interleukin 12- & 23-blocking drugs, an interleukin 17-blocking drug & a small-molecule drug that inhibits phosphodiesterase 4.
Tweaking the Methodology to Improve Clinical Relevancy?
(click for larger image)
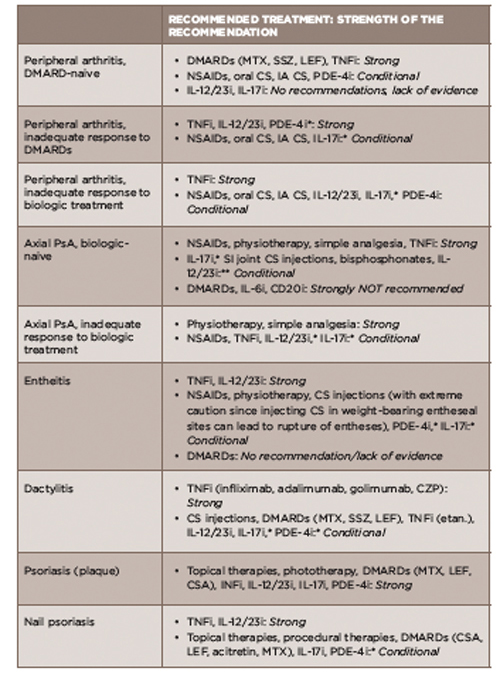
TABLE 2: Treatment Recommendations for PsA Based on Disease Domain
*recommendations based on data published in abstract form only
**recommendation based only on data from small, open-label, proof-of-concept trials published only in abstract form
Acronyms: DMARD, disease-modifying antirheumatic drug; MTX, methotrexate; SSZ, sulfasalazine; LEF, leflunomide; TNFi, tumor necrosis factor inhibitor; NSAIDs, nonsteroidal anti-inflammatory drugs; oral CS, chondroitin sulfate; IA CS, intra-articular corticosteroid; PDE-4i, phosphodiesterase 4 inhibitor; IL-12/23i, interleukin 12/23 inhibitors; IL-17i, interleukin 17 inhibitor; SI, sacroiliac joint; IL-6i, interleukin 6 inhibitor; CD20i, CD 20 inhibitor; CZP, certolizumab pegol; etan, etanercept
Source: Adapted from Table 2, Reference 1.


