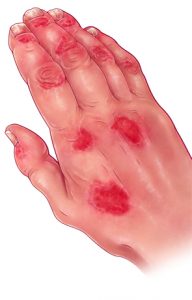
This illustrates a patient with psoriasis accompanied by arthritis and other joint problems. Aside from the appearance of red skin lesions and joint inflammation, changes in fingernails and dactylitis (swelling of fingers that create a sausage-like appearance) are characteristic of psoriatic arthritis.
Evan Oto/Science Source
In May, the Group for Research and Assessment of Psoriasis and Psoriatic Arthritis (GRAPPA) published its recommendations for the treatment of psoriatic arthritis (PsA).1 The updated recommendations represent advances in drug development and availability since previous recommendations published in 2009, as well changes in treatment paradigms and the importance of associated aspects of the disease.2
“The new list of treatment recommendations provides a way for clinicians to keep up with the literature in a field that rapidly evolves,” says Arthur Kavanaugh, MD, a coauthor of the recommendations, and professor of medicine in the Division of Rheumatology, Allergy and Immunology at the University of California–San Diego, La Jolla, Calif. “The recommendations also reflect a growing appreciation of other aspects of the disease, such as the importance of comorbidities, which previous recommendations did not address,” he adds.
Along with including the importance and treatment of comorbidities associated with PsA, the new recommendations include three new classes of drugs that have come to the market since the 2009 recommendations. These include interleukin 12- and 23-blocking drugs, an interleukin 17-blocking drug and a small-molecule drug that inhibits phosphodiesterase 4.
“The old recommendations had the tumor necrosis factor inhibitors as the only biologics,” says Atul Deodhar, MD, professor of medicine, and medical director of the Rheumatology Clinic, as well as Immunotherapy Infusion Center, Division of Arthritis & Rheumatic Diseases, Oregon Health & Science University, Portland, Ore. “Rheumatologists may not be aware of the appropriate place in the treatment plan for these new class of drugs, so these recommendations are needed to help guide them on how they can be used and when they can be used.”
Updated Recommendations
The 2015 updated recommendations were developed by a panel of GRAPPA rheumatologists, dermatologists and patients with PsA, which reviewed the current literature on treatment of key domains of PsA, including arthritis, spondylitis, enthesitis, dactylitis, skin disease and nail disease. New to this update was the inclusion of a review to identify pertinent comorbidities associated with PsA and their effect on treatment.
(click for larger image)
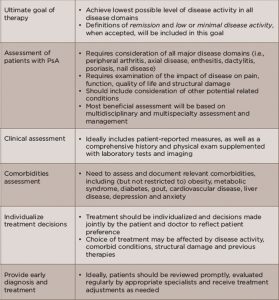
TABLE 1: Overarching Principles for PsA Management
Source: Adapted from Table 1, References 1 and 3.
Prior to developing the list of recommendations, the group drafted a set of overarching principles on the management of PsA that were agreed upon by a near consensus (≥80%) (see Table 1).1,3
Using a modified Grading of Recommendations, Assessment, Development and Evaluation (GRADE) process, the panel then developed treatment recommendations for each disease domain (see Table 2) (as well as comorbidities) and a schema that incorporated the overarching principles.1
Dr. Kavanaugh emphasized that these treatment recommendations are meant to help clinicians optimize their care for patients with PsA. The recommendations do not, however, provide clear guidance on exactly when to use each agent.
“I think that when recommendations come out there is a hope or aspiration that they may be more specific than they can be,” he says, “meaning that along with listing the recommended therapies, the specifics on when to use which drug first in a given patient [would] also [be] provided.”
“That would be nice, but we don’t have that information, so the clinician is left with clinical experience and patient choice,” he adds. “So that is partially why more patient involvement is included in these guidelines compared to older guidelines.”
Dr. Deodhar emphasizes that clinicians will want to know how to use these new drugs, whether as first-line treatment or as second-line treatment after anti-TNF inhibition failure. Given the lack of head-to-head studies on comparative efficacy of the drugs, he says that clinicians can look at their comparative safety profiles.
For example, when comparing the safety of methotrexate to the new small-molecule drug, apremilast, he says apremilast seems to be extraordinarily safe except for stomach upset and diarrhea and appears not to be associated with the more severe side effects of liver function abnormalities or significant immunosuppression.
“However, we have 40 years of experience with methotrexate and only three years of experience with apremilast, so the side effects may appear later,” he cautions.
Of course, cost is also an issue, because the cost of the newer agents is extraordinary, he says.
The new recommendations include three new classes of drugs that have come to the market since the 2009 recommendations. These include interleukin 12- & 23-blocking drugs, an interleukin 17-blocking drug & a small-molecule drug that inhibits phosphodiesterase 4.
Tweaking the Methodology to Improve Clinical Relevancy?
(click for larger image)
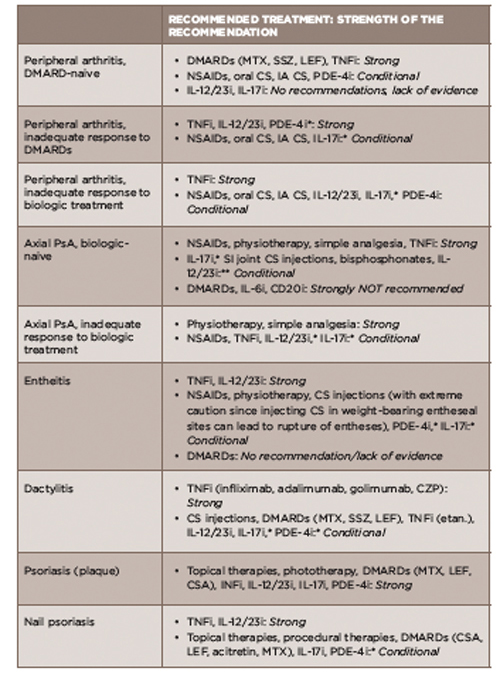
TABLE 2: Treatment Recommendations for PsA Based on Disease Domain
*recommendations based on data published in abstract form only
**recommendation based only on data from small, open-label, proof-of-concept trials published only in abstract form
Acronyms: DMARD, disease-modifying antirheumatic drug; MTX, methotrexate; SSZ, sulfasalazine; LEF, leflunomide; TNFi, tumor necrosis factor inhibitor; NSAIDs, nonsteroidal anti-inflammatory drugs; oral CS, chondroitin sulfate; IA CS, intra-articular corticosteroid; PDE-4i, phosphodiesterase 4 inhibitor; IL-12/23i, interleukin 12/23 inhibitors; IL-17i, interleukin 17 inhibitor; SI, sacroiliac joint; IL-6i, interleukin 6 inhibitor; CD20i, CD 20 inhibitor; CZP, certolizumab pegol; etan, etanercept
Source: Adapted from Table 2, Reference 1.
Although lauding the recommendations for nicely condensing the past seven years of advances, Dr. Deodhar suggests that using the GRADE (Grading of Recommendations Assessment, Development and Evaluation) methodology without modifications (that were used in the current recommendations) may make future recommendations more relevant to clinical practice.
When using the GRADE methodology, investigators first look at questions of interest to clinical outcomes to generate the types of studies to include in the systematic review of the literature. These are called PICO (population, information, comparator and outcome) questions. Such an approach focuses first on the important clinical questions of interest to rheumatologists, and, therefore, the recommendations are tailored toward specific clinical situations.
“My suggestion is that next time [the recommendations are updated], the investigators start with the PICO questions and address what the important clinical questions are for rheumatologists,” he says. “Then, base the recommendations on that.”
Although the current recommendations did use the GRADE methodology, it used a modified form in which the investigators began with overarching questions on what data were available on the treatment of the various domains of PsA, followed by a literature search to find data on the efficacy and safety of these data. According to Dr. Kavanaugh, although the GRADE methodology is quite useful for very limited questions that fit either/or treatment decisions, it is less helpful with heterogeneous diseases, such as PsA, that have multiple potential therapies,because such a methodology would generate myriad questions that would be too numerous to tackle.
Live Document
Another update to the current recommendations may not be too far off. Both Drs. Kavanaugh and Deodhar emphasize that the rapid evolution of changes in the understanding and treatment of PsA mandates the need to update these recommendations continuously.
“In such a rapidly changing field, it is good to be on the lookout for new recommendations, because even though the current recommendations are brand new, there is already new information on the short horizon that will be useful,” says Dr. Kavanaugh.
Calling the recommendations a live document, Dr. Deodhar says he hopes the recommendations will be updated on an ongoing basis to meet the rapid pace of drug discovery and new questions that arise for the clinician on how to implement these drugs.
One pressing issue already, he says, is the need to provide clinicians with guidance on how to use the recently approved first biosimilar to treat PsA.
Dr. Kavanaugh acknowledges the need for continual updating to meet the ongoing challenge presented by the rapid pace of change. “The challenge is that there are newer therapies and treatment strategies that are being tested in ongoing studies, and there is a lag time between when the results are presented at an international meeting and when they are published,” he says. He says part of the challenge is to adhere to producing evidence-based guidelines while not spending too much time and effort on a document that will be outdated soon after it is published.
Given this, Dr. Kavanaugh says the next version of the recommendations should probably start soon.
Mary Beth Nierengarten is a freelance medical journalist based in Minneapolis.
References
- Coates LC, Kavanaugh A, Mease PJ, et al. Group for Research and Assessment of Psoriasis and Psoriatic Arthritis 2015 Treatment Recommendations for Psoriatic Arthritis. Arthritis Rheumatol. 2016 May;68(5):1060–1071.
- Richlin CT, Kavanaugh A, Gladman DD, et al. Treatment recommendations for psoriatic arthritis. Ann Rheum Dis. 2009 Sep;68(9):1387–1394.
- 2015 Treatment Recommendations for Psoriatic Arthritis. The Rheumatologist [online]. 2016 Apr 28. http://www.the-rheumatologist.org/article/2015-treatment-recommendations-psoriatic-arthritis.


