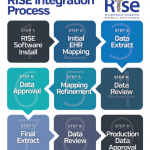
E. William St.Clair, MD, the ACR’s immediate past president, wrote in the June 16, 2015, issue of The Rheumatologist that the registry was designed to become a “best-in-class resource to help rheumatologists, rheumatology health professionals and researchers manage patient populations, improve patient care and navigate the ever-changing healthcare environment.” RISE gives physicians real-time access to data and customized quality-improvement quires and reports.
The RISE Registry collects demographic, medication, and lab data, as well as data on disease activity and functional status. The ACR is responsible for oversight and monitoring of the registry and its information integrity. The system allows participants to run both standard and custom queries for one’s own patient population and to compare that patient population with the aggregate of the patients in the registry.
The registry is HIPAA compliant, and the ACR will not have access to a patient’s protected health information. Any patient protected health information is stored separately. The only data used by the ACR or third parties for research or other purposes will be aggregate data, not individualized data.
The participation agreement includes both a data use agreement and a business associate agreement. The ACR does not require workflow changes to participate in the registry; it is acceptable if there are data that a rheumatologist practice is not capturing. Additionally, participation includes working with the ACR’s registry partner, FIGmd, over a series of conference calls to review and refine data mapping to the registry. More information about RISE is available at http://www.rheumatology.org/I-Am-A/Rheumatologist/Registries/RISE/RISE-FAQs.
Additional Registries
The Syndromic Surveillance Registry is operated by individual states. Please contact your state’s public health department for specific details.
The CDC offers a list of Immunization Registries by state. These include population-based data on immunization doses administered by participating providers. For more information, see http://www.cdc.gov/vaccines/programs/iis/contacts-registry-staff.html.
Regardless of which registries you choose, the date to remember is Feb. 29, 2016, or you’ll face financial penalties for not complying with the requirement.
Kathy L. Holliman, MEd, is a medical writer based in Beverly, Mass.