ACR CONVERGENCE 2020—The results of a post-authorization study comparing the cardiovascular safety of febuxostat vs. allopurinol were presented in a late-breaking abstract session at the ACR’s fully virtual annual meeting on Monday, Nov. 9.
Cardiologist Thomas MacDonald, MD, FRCP, MBChB, clinical professor (teaching and research) of molecular and clinical medicine, University of Dundee School of Medicine, Scotland, U.K., presented the results from the so-called FAST study: “We did a prospective, randomized, open-label, blinded endpoint (PROBE) trial of febuxostat vs. allopurinol in patients with chronic gout in the U.K., Denmark and Sweden,” he said.1
“As you all know, febuxostat and allopurinol are uric acid-lowering agents,” he said. Concerns about the cardiovascular safety of febuxostat have been raised, so the European Medicines Agency (EMA) recommended a post-authorization study. “The FDA [U.S. Food & Drug Administration] had also requested such a study, the CARES study, and it actually reported increased cardiovascular and all-cause deaths leading to an FDA warning and changes in treatment recommendations.”2
The Study
Between Dec. 20, 2011, and Jan. 26, 2018, 6,128 patients (mean age 71; 85.3% male; 33.4% with prior established cardiovascular disease) were randomized to receive allopurinol (n=3,065) or febuxostat (n=3,063), and were followed up for a median of four years, during which 5.5% and 6.2%, respectively, withdrew from all follow-up. “So this was really a long-term study,” he said.
Eligible patients were 60 years or older, were already being treated with allopurinol and had at least one additional cardiovascular risk factor. “We excluded strokes and MIs in the past six months, and severe heart failure,” Prof. MacDonald said.
“Allopurinol was increased—we titrated people up—if they weren’t at target at screening, and we did that by increasing the allopurinol dose at 100 mg every two weeks until they reached target or the maximum recommended dose … to achieve a serum uric acid level of less than 0.357 mmol/L [<6 mg/dL],” he said.
Patients were randomly assigned to continue allopurinol (at the optimized dose) or to start febuxostat at a dose of 80 mg daily, increasing to 120 mg, if necessary, to achieve a serum uric acid level of <0.357 mmol/L.
The primary outcome was the composite of hospitalization for non-fatal myocardial infarction/biomarker positive acute coronary syndrome, non-fatal stroke or cardiovascular death. This was ascertained via clinician or patient reports and by national healthcare records, post-mortem and death certification. The hazard ratio (febuxostat vs. allopurinol) in a Cox proportional hazards model was assessed for non-inferiority (limit of 1.3) in an on-treatment analysis and then by intention to treat.
In both the on-treatment (OT) and intention-to-treat (ITT) analyses, febuxostat was non-inferior to allopurinol for incidence of the primary endpoint (OT analysis: febuxostat 172 patients [1.72 events per 100 patient years]; allopurinol 241 patients [2.05 events per 100 patient years]; hazard ratio 0.85 [95% CI 0.70–1.03], P<0.001; ITT analysis: febuxostat 256 patients [2.05 events per 100 patient years], allopurinol 285 patients [2.29 events per 100 patient years]; hazard ratio 0.89 [95% CI 0.75–1.06], P<0.001).
Two hundred twenty-two (7.2%) patients died in the febuxostat group, compared with 263 deaths (8.6%) in the allopurinol group.
“Because you’re all rheumatologists in here, you all want to know about gout flares,” said Prof. MacDonald. “These weren’t really adjudicated, but patients in both groups had at least one gout flare”: 1,017 patients in the febuxostat group (event rate 17.95 per 100 patient-years) and 1,044 patients in the allopurinol group (event rate 19.85 per 100 patient-years). “But, of course, there’s no placebo group in here, so we don’t know the effectiveness of either of these agents in preventing these flares,” he said.
The study concluded that febuxostat was non-inferior to allopurinol therapy for the primary cardiovascular outcome. “In contrast to previous studies there was no evidence of increased mortality with febuxostat,” he said, “and we think regulators should review febuxostat licensing restrictions.”
In response to a question about the quality of the data from the audience, Prof. MacDonald said, “The concept that we missed deaths or hospitalizations is pretty unlikely. … The records are pretty complete, and we had pretty good follow-up.”
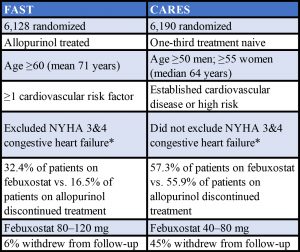 * New York Heart Association Classification. Stage 3: Marked limitation in activity due to symptoms, even during less-than-ordinary activity, (e.g., walking short distances [20–100 m]). Comfortable only at rest. Stage 4: Severe limitations. Experiences symptoms even while at rest. Mostly bedbound patients.
* New York Heart Association Classification. Stage 3: Marked limitation in activity due to symptoms, even during less-than-ordinary activity, (e.g., walking short distances [20–100 m]). Comfortable only at rest. Stage 4: Severe limitations. Experiences symptoms even while at rest. Mostly bedbound patients.
Sources: Adapted from a table presented by Prof. MacDonald and from the Specifications Manual for Joint Commission National Quality Measures (v2018A)
Keri Losavio is the editor of The Rheumatologist.
Study Disclosures: The study was funded by Menarini to fulfil an EMA regulatory commitment; however, Menarini played no part in the running of this study. Menarini received financial support from Ipsen and Teijin Pharma Ltd. for this study. The University of Dundee was the legal sponsor, and received funds for research from Amgen, Astellas, AstraZeneca, GSK, Menarini, Pfizer, Servier, Shire and Takeda. Prof. MacDonald has received speaker or consultancy fees from Novartis, Takeda, Servier, Shire, Astellas, Menarini and AstraZeneca.
References
- MacDonald T, Mackenzie I, Nuki G, Ford I. Long term cardiovascular safety of febuxostat and allopurinol in patients with chronic gout: The febuxostat versus allopurinol streamlined Trial (on Behalf of the FAST Investigators) [abstract]. Arthritis Rheumatol. 2020 Oct;72(suppl 10).
- White WB, Saag KG, Becker MA, et al. Cardiovascular safety of febuxostat or allopurinol in patients with gout. N Engl J Med. 2018 Mar 29;378(13):1200–1210.