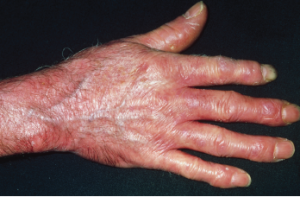
Red, shiny, tight skin on the hand due to scleroderma.
Dr P. Marazzi / Science Source
“You have systemic sclerosis,” says the physician.
Other somber words patients may hear the day they learn their diagnosis include, “rare, chronic, no treatment.” But a ray of hope could soon enter those exam rooms thanks to research conducted by a team from the UK.
Rizgar A. Mageed, PhD, FRCP, FRCPath, is professor of experimental immunology at the Centre for Experimental Medicine and Rheumatology at Queen Mary University of London. His most recent study, “Association of Defective Regulation of Autoreactive Interleukin-6-Producing Transitional B Lymphocytes with Disease in Patients with Systemic Sclerosis,” appears in the March 2018 edition of Arthritis & Rheumatology.1
Dr. Mageed discusses what he’s learned with The Rheumatologist.
B Lymphocytes

Dr. Mageed
“Systemic sclerosis (SSc) is a disease with no cure and a high rate of mortality,” Dr. Mageed says. “One significant feature is fibrosis that emanates from an excessive deposition of proteins in the extracellular matrix of the skin and visceral organs that ultimately damage the skin, cardiovascular system and lungs. The disease is associated with autoantibodies … so in scleroderma patients, the immune system produces antibodies that don’t kill pathogens but attack self-proteins.
“The important thing about autoantibodies in patients with SSc is that some have skin involvement, others have lung involvement [and others involve] the cardiovascular system … and the type of autoantibodies seems related to the [patient’s] distinct symptoms and organ involvement,” Dr. Mageed continues. “The role of these antibodies in causing the disease is that they appear prior to the disease onset. But we know little about the origin of autoantibody-producing B lymphocytes, [or] why they are activated. [In our study], we set out to determine the mechanisms that could contribute to the generation of disease-causing B cells, and to find out more about how B cells contribute to disease in SSc patients.
“Some clinicians have tried using biological agents that eliminate all B lymphocytes, finding that some patients benefit clinically but others do not,” he says. “This is further confirmation that B lymphocytes do play an important disease-causing role in scleroderma.
“In healthy individuals,” explains Dr. Mageed, “immune cells are well regulated, [so they] are not making autoantibodies because of immunological tolerance, the process whereby healthy individuals make lymphocytes and those that seem risky are deleted. In SSc, however, patients have B lymphocytes that recognize self-proteins and make autoantibodies.”
Checkpoints Gone Awry
Dr. Mageed says, “B lymphocytes are produced in the bone marrow and go through a lengthy maturation process. Before leaving the bone marrow, they undergo a rigorous immunological tolerance process so that nearly all B lymphocytes that can recognize self-proteins are killed off. Some B lymphocytes manage to get through, however, and thus leave the bone marrow and get into the blood. But the body has anticipated this kind of issue and has backup systems. Whatever self-reactive B lymphocytes don’t get eliminated in the bone marrow will be removed before they mature into adult B cells.
“In a previous study,” he says, “we found that because they transition from bone marrow to the periphery, these cells undergo four stages of maturation and regulation. We determined that between stages one and two, the majority of potentially dangerous B lymphocytes are removed. In SSc patients, however, there is a defect in the checkpoint between stages one and two.
“So our conclusion is that patients with SSc have a wiring system of B lymphocytes that is problematic—the signaling inside these cells has gone awry,” Dr. Mageed says. “They do indeed receive the death signal, but they do not respond.
“These B lymphocytes in SSc patients also produce excess amounts of interleukin (IL) 6 and IL-8,” he says. This is important because IL-6 promotes, while IL-8 augments, inflammation. Combine this with the production of self-reactive antibodies by B lymphocytes and it leads to the pathology we associate with SSc.
‘Patients with SSc have a wiring system of B lymphocytes that is problematic—the signaling inside these cells has gone awry.’ —Dr. Mageed
Tailor-Made Drugs
“Our next step,” says Dr. Mageed, “is to determine what distinguishes bad B cells in SSc patients from normal B cells, which is complicated [because] there are many unknowns. It may be in the nature of the proteins they express. A cell expresses thousands of proteins, each of which functions to keep the cell alive and ensure it functions properly. We must examine all proteins expressed and compare them with the good B cells. Is there a particular protein expressed on the surface of, or even inside, the cell that makes the bad B lymphocytes different from the healthy ones? Once we know that, it’s a fairly straightforward procedure and we can formulate new types of medications—biological therapies.
“Biologics are directed at one specific target, unlike aspirin, paracetamol, steroids, etc., which act systemically,” Dr. Mageed says. “At this point, there are thousands of biologics being successfully used for specific diseases, so this is a promising realm for SSc patients as well. One particular drug, an anti-B lymphocyte, binds to B lymphocytes and kills them—but it also kills the good cells. Our goal is to design a specific biological therapy that will target [only] the bad B cells. In roughly two to three years, we may have identified the proteins that are differentially expressed in bad B lymphocytes; then we can begin making the biologics to target them. This is the foundation of designer therapy.”
Elizabeth Hofheinz, MPH, MEd, is a freelance medical editor and writer based in the greater New Orleans area.
Reference
- Taher TE, Ong VH, Bystrom J, et al. Association of defective regulation of autoreactive interleukin-6–producing transitional B lymphocytes with disease in patients with systemic sclerosis. Arthritis Rheumatol. 2018 Mar;70(3):450–461.

