We are fortunate to have clinical practice guidelines for the management of psoriasis and psoriatic arthritis (PsA) from multiple organizations to help navigate today’s rapidly evolving therapeutic landscape. We are further fortunate to have multiple specialists to manage these conditions: rheumatologists and dermatologists. However, multiple guidelines, multiple drugs and multiple specialists can create a paradox of choice, with an abundance of options creating anxiety in the chooser.1 (For an overview of all the options available to treat psoriatic arthritis, see “Psoriatic Arthritis Treatment Update.”)
We are fortunate to have clinical practice guidelines for the management of psoriasis and psoriatic arthritis (PsA) from multiple organizations to help navigate today’s rapidly evolving therapeutic landscape. We are further fortunate to have multiple specialists to manage these conditions: rheumatologists and dermatologists. However, multiple guidelines, multiple drugs and multiple specialists can create a paradox of choice, with an abundance of options creating anxiety in the chooser.1 (For an overview of all the options available to treat psoriatic arthritis, see “Psoriatic Arthritis Treatment Update.”)
The goal of treatment in psoriasis is simple: clear skin. In psoriatic arthritis, the goal is no more complicated: clear skin, happy joints. But with so many ways to meet the same goals, where should we start? In this article, we explore how the dermatologist selects a therapy, and how the rheumatologist and dermatologist might best collaborate to achieve the same end.
Clinical Practice Guidelines
In 2018, the American Academy of Dermatology and National Psoriasis Foundation released joint guidelines for the management and treatment of psoriasis with biologic therapies. These guidelines notably did not identify a preferred drug class as firstline therapy.2
The same year, the ACR and the National Psoriasis Foundation released a joint guideline for the treatment of PsA. Tumor necrosis factor (TNF) inhibitors were conditionally recommended as first-line biologic agents for PsA.3
In 2019, EULAR updated its guidelines for the pharmacologic management of PsA. Notably, the initial biologic disease-modifying anti-rheumatic drug (bDMARD) selection recommendation was expanded to include TNF inhibitors and anti-interleukin (IL) 17 or anti-IL-12/23 medications, as opposed to the 2015 guidelines, which endorsed TNF inhibitors for the firstline treatment of PsA.4
Guidelines from the Group for Research and Assessment of Psoriasis and Psoriatic Arthritis (GRAPPA) in 2015 supported the use of TNF inhibitors, anti-IL-17 or
anti-IL-23 drugs as first-line treatments, depending on the clinical scenario. Updated GRAPPA guidelines are in progress, with anticipated publication in 2021.5
Dermatologist Perspective
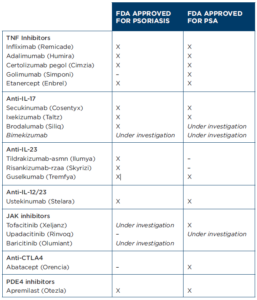
Although well intentioned, this alphabet soup of guidelines can be overwhelming, as can the laundry list of available therapeutics (see Figure 1). Colby C. Evans, MD, a dermatologist in Austin, Texas, and a board member and former chair of the National Psoriasis Foundation, helps us understand drug selection from the dermatologist’s perspective. He comments on the role of methotrexate, when he adds a biologic, how he selects a biologic and what he is looking for in a co-managing rheumatologist.
“There is a still a role for methotrexate in the management of psoriasis, but it is getting smaller,” says Dr. Evans. “Methotrexate can still be used as an inexpensive first-line agent in certain situations. It is also often used as an adjunct agent to biologics to improve efficacy and possibly inhibit anti-drug antibodies. My perception is that dermatologists use it less often than other specialists, and that use continues to decrease as more biologic and oral options become available.”
In terms of when he reaches for a biologic to treat psoriasis, Dr. Evans says, “Biologics are commonly firstline agents in the modern treatment of psoriasis, especially severe psoriasis that either covers a large body surface area, or affects sensitive sites like the hands, feet, genitals or scalp. They can also be added when topicals, ultraviolet B, and oral agents have failed. It is generally more based on patient specific factors than an inflexible algorithm.”
As in PsA, there is no clear firstline biologic choice for the management of psoriasis. When he selects a therapy, Dr. Evans shares, “It is certainly patient specific, and most of the currently available biologics have a place in first-line treatment. Many dermatologists base their first decision point on whether the patient has psoriatic arthritis, in which case a TNF inhibitor or IL-17 inhibitor may be the first choice. Similarly, patient risk tolerance, comorbidities and payer factors may also influence the choice.”
Do TNF Inhibitors Still Play a Role?
With a veritable explosion of psoriasis and PsA bDMARDs within the past few years, clinical practice guidelines are struggling to keep up. Fortunately, network meta-analyses are providing some insight into comparative drug efficacy, and head-to-head randomized controlled trials are emerging and will inform future
practice guidelines.
The Cochrane Database of Systematic Reviews is conducting a living network meta-analysis of systemic pharmacological treatments for chronic plaque psoriasis, last updated in April 2021.6 The study includes 158 randomized controlled trials of systemic treatments in adults with moderate to severe plaque psoriasis or psoriatic arthritis with concomitant moderate to severe plaque psoriasis. The primary outcome was clear or almost clear skin—specifically, a Psoriasis Area and Severity Index (PASI) 90 at induction phase.
Interestingly, the authors concluded that compared with placebo, anti-IL-17 and anti-IL-23 inhibitors were the most effective induction treatments for clearing skin; infliximab was also very effective, whereas other TNF inhibitors were notably less so. Because the review was limited to induction therapy, the authors were unable to evaluate longerterm outcomes.
Findings from a network meta-analysis published in JAMA Dermatology by Armstrong et al. in February 2020 had similar findings, with anti-IL-17 and anti-IL-23 therapies clearing skin most effectively and PASI 90 rates around 70%.7 The PASI 90 rate for TNF inhibitors and anti-IL-12/23 (ustekinumab) ranged from 17.9 to 43.9%.

Dr. Evans
Dr. Evans acknowledges the limitations of data like these, noting that “head-to-head blinded trials remain our best tool for understanding relative efficacy in psoriasis, but these are unfortunately expensive and difficult.” However, he does agree that “database analyses can certainly be useful tools, and these seem to confirm the common belief among dermatologists that IL-17 and IL-23 agents are the most broadly effective for the skin.”
He continues to stress that, “this being said, the treatment of psoriasis is highly individualized based on patient factors, including common comorbidities like PsA, inflammatory bowel disease and coronary artery disease.”
As for TNF inhibitors, Dr. Evans does think there is a continued role for them in the treatment of psoriasis, saying, “They remain good choices in patients with substantial PsA and are often required by payers as part of a step therapy scheme. They also continue to be used for other indications; for example, adalimumab for hidradenitis suppurativa, or off-label for cutaneous sarcoid and granuloma annulare.”
The Dermatologist’s Ideal Rheumatologist
What does Dr. Evans hope for in a co-managing rheumatologist? “Good communication is key when collaborating in the care of a psoriasis patient,” he says. “I generally prefer for one physician to be the ‘quarterback,’ usually based on whether the skin or the joints are the more pressing issue for that specific patient. If the joints are more severe, I generally prefer the rheumatologist manage the biologics and orals, while I can add topical or ultraviolet therapies as adjuncts.”
A Paradox of Choice?
In 2021, multiple guidelines, multiple drugs and multiple specialists may indeed create a paradox of choice; however, the many choices we now have for the treatment of psoriasis and PsA allow for a bespoke style of care, tailored to the individual patient. The armamentarium of bDMARDs in psoriasis and PsA exemplifies when more is more.
So call the co-managing dermatologist, embrace the guideline gray area, and personalize drug selection to the patient sitting in front of you. Their skin and joints
will thank you.
Samantha C. Shapiro, MD, is an academic rheumatologist and an affiliate faculty member of the Dell Medical School at the University of Texas at Austin. She received her training in internal medicine and rheumatology at Johns Hopkins University, Baltimore. She is also a member of the ACR Insurance Subcommittee.
References
- Schwartz B. The Paradox of Choice: Why More Is Less. New York: Ecco Press, an imprint of Harper Collins; 2004.
- Menter A, Strober BE, Kaplan DH, et al. Joint AAD-NPF guidelines of care for the management and treatment of psoriasis with biologics. J Am Acad Dermatol. 2019 Apr; 80(4):1029–1072.
- Singh JA, Guyatt G, Ogdie A, et al. 2018 American College of Rheumatology/National Psoriasis Foundation guideline for the treatment of psoriatic arthritis. Arthritis Care Res (Hoboken). 2019 Jan;71(1):2–29.
- Gossec L, Baraliakos X, Kerschbaumer A, et al. EULAR recommendations for the management of psoriatic arthritis with pharmacological therapies: 2019 update. Ann Rheum Dis. 2020 Jun; 79(6):S700–S712.
- Coates LC, Corp N, van der Windt DA, et al. GRAPPA treatment recommendations: An update from the 2020 GRAPPA annual meeting. J Rheumatol. 2021 Jun;97:65–66.
- Sbidian E, Chaimani A, Garcia-Doval I, et al. Systemic pharmacological treatments for chronic plaque psoriasis: A network meta‐analysis. Cochrane Database Syst Rev. 2021 Apr 19;4(4):CD011535.
- Armstrong AW, Puig L, Joshi A, et al. Comparison of biologics and oral treatments for plaque psoriasis: A Meta-analysis. JAMA Dermatol. 2020 Mar 1;156(3):258–269.