The high incidence of premature or early onset atherosclerotic cardiovascular disease (ASCVD) is one of the most compelling and, as yet, incompletely appreciated clinical challenges facing rheumatologists caring for patients with systemic lupus erythematosus (SLE).
Improved therapies have reduced the likelihood that patients with systemic autoimmunity will succumb to their primary disease, and as a result, the awareness of an increased cardiac risk in these patients has slowly grown. At Hospital for Special Surgery in New York City, recognition that young women followed for lupus were suffering heart attacks led us to design a study to formally examine the prevalence and associated clinical features of ASCVD in this population.
The presence of ASCVD in lupus patients was initially identified in two reports from the 1970s: an autopsy study showing atherosclerotic narrowing of coronary arteries in relatively young SLE patients and a clinical study demonstrating a bimodal pattern of mortality from SLE with late deaths due to myocardial infarction.1,2
During the next 25 years, evidence accumulated to confirm that ASCVD is an important cause of death and hospitalization in the SLE population. In series reported after 1975, 6% to 45% of deaths in SLE patients were due to coronary artery disease—many in premenopausal women—and the prevalence of clinically manifest ischemic disease (myocardial infarction [MI] or angina) ranged from 6% to 20%.3 In the Pittsburgh SLE Registry, women age 35 to 44 were more than 50 times likelier to have an MI than historical controls from the Framingham population.4
While classical risk factors for cardiovascular disease (age, hypertension, smoking, family history, diabetes mellitus, and hypercholesterolemia) are operative in SLE patients, the risk for MI conferred by SLE increased 10.1-fold in one study after controlling for these factors, suggesting that SLE itself, and/or its treatment, were primarily responsible for ASCVD.5 Although there was consensus that clinical events due to ASCVD appeared prematurely and more frequently in SLE, their absolute number was small and the prevalence of underlying preclinical atherosclerosis was not known and its causes not understood.
Rheumatology-Cardiology Collaboration
At the same time rheumatologists were recognizing that ASCVD was more common in patients with systemic autoimmunity, cardiologists and vascular biologists began to appreciate that immunological processes participate in atherogenesis. By the 1990s, evidence emerged for immune triggers of cardiovascular disease—classical risk factors were not the full story—and basic and clinical science journals were replete with studies describing the relationship between inflammation and atherosclerosis.6-8
Despite these advances, much was still unknown when we began our studies seven years ago. What was the prevalence of atherosclerosis in SLE patients in relation to a control group? What elements of SLE affected risk? What were the contributions of conventional risk factors, disease severity, and treatment? Did therapy of SLE increase or decrease the risk of ASCVD? How and in whom should we intervene?
To devise a study that would address these questions, we assembled a group of rheumatologists and cardiologists, including Mary J. Roman, MD, professor of medicine at Weill Medical College of Cornell University, who agreed to become the lead cardiologist in the project. Because event rates—myocardial infarction and angina—are relatively low in this young population, and because it was impractical to recruit and study thousands of lupus patients, we needed a surrogate marker to define the prevalence of preclinical atherosclerosis.
Atherosclerosis was associated with longer disease duration, higher damage scores, and less aggressive immunosuppressive therapy. This finding argues that chronic inflammation is atherogenic in SLE.
A few months after our exploratory meetings in 1997 the team from Hospital for Special Surgery was invited to a conference sponsored by the National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS) and the National Heart, Lung, and Blood Institute to discuss cardiovascular disease in SLE patients. This interdisciplinary group also concluded that the classical approach—one that involved hard clinical endpoints for atherosclerosis and enormous populations to define prevalence and risk factors—although preferable, was not feasible, and that markers for preclinical disease were required. We selected ultrasound evidence of carotid atherosclerosis.
Safe and Accessible Imaging
Ultrasound examination of the extra-cranial carotid arteries to detect the presence of discrete atherosclerotic plaque (established atherosclerosis) and to quantify intimal-medial thickness (a measure of “early” or diffuse atherosclerosis) is a safe, accurate, and sensitive method for detecting atherosclerosis in large populations. Carotid vascular disease is strongly correlated with the presence of atherosclerotic disease in other vascular systems, particularly the coronary bed, and has been shown to be an independent predictor of adverse clinical outcomes, most commonly myocardial infarction.9,10 Moreover, carotid ultrasonography, a non-invasive imaging modality, was both safer and less expensive that many of the other techniques we considered.
In 2000, we began the first case-control study to assess the prevalence, magnitude, and determinants of atherosclerosis in SLE in a population-based sample. We recruited, at time of outpatient visits, more than 200 sequential non-hospitalized patients with SLE. Patients were enthusiastic to participate because they were able to have an examination by a skilled cardiologist, Dr. Roman, and non-invasive imaging, as well as blood tests to assess their ASCVD risk profile.
Many patients expressed an interest in the outcome of the study and appreciated being able to contribute to increasing knowledge of their disease. In part for this reason, each study subject received reprints of papers describing the results of the studies in which they participated. Similarly, doctors were grateful for additional information obtained at no cost to the patient which, in some cases, prompted changes in therapy. They, too, valued the opportunity to be part of a scientific study.
Correlation between Lupus and Atherosclerosis
Patients underwent carotid ultrasonography and echocardiography. (See Figure 1, page 14.) They were assessed for traditional cardiovascular disease risk factors, SLE disease activity and damage, treatment history, autoantibody profile, and the presence of inflammatory mediators. Each SLE patient was individually matched to a control subject based on age, gender, race, and blood pressure status. Control subjects were drawn from individuals participating in an NIH-funded study at our institution that used a similar imaging protocol to examine the impact of job strain, aging, and hypertension on preclinical cardiovascular disease. Because a large control population had already been evaluated, we were able to complete our study more quickly and leverage NIH funding for both projects.
When we analyzed the entire population (lupus and controls), we found that only the presence of lupus, age, and (equivocally) cholesterol level were independent risk factors for atherosclerosis.11 Atherosclerosis was significantly increased in lupus (carotid plaque in 37.1% of SLE patients versus 15.2% of controls), most strikingly in younger individuals. (See Figure 2, page 14.) This increase was not attributable to traditional cardiovascular risk factors or corticosteroid therapy. In fact, SLE was the strongest independent correlate of carotid atherosclerosis among patients and control subjects comparable with regard to traditional risk factors.
What were the unique characteristics of SLE patients with carotid plaque? Clinical features and autoantibody specificities were different in SLE patients with and without plaque. We saw two distinct disease patterns: one characterized by prolonged, smoldering disease with high damage scores, limited production of autoantibodies, and more prevalent atherosclerosis; the other with a wider spectrum of autoantibody specificities, a history of more aggressive immunosuppressive therapy, and less prevalent plaque.11
We found that atherosclerosis was associated with longer disease duration, higher damage score, and less aggressive immunosuppressive therapy. This finding argues that chronic inflammation is atherogenic in SLE. While circulating markers of inflammation were high in many SLE patients, there were no significant differences in levels of these markers between patients with and without plaque.
Unresolved Issues
- What are the direct links between chronic inflammation and the initiation and progression of atherosclerosis?
- Are certain patients at greater risk than others and, if so, how can they best be identified?
- Are other chronic inflammatory diseases associated with premature vascular aging?
- Should patients—particularly young patients—be screened for premature atherosclerosis and, if so, by what means?
- Should chronic inflammatory diseases be considered ASCVD equivalents, similar to diabetes mellitus?
Whereas C-reactive protein, sICAM, and sCD40L are associated with increased risk of cardiovascular events in the general population, these markers appear to lose discriminatory power for predicting adverse cardiovascular outcome in conditions like lupus, where inflammatory molecule levels are elevated compared to the general population. Not surprisingly, levels of markers of inflammation were abnormal in many patients with SLE, and longitudinal, rather than cross-sectional, studies are more likely to define their relation to the development and progression of atherosclerosis.
RA and Cardiovascular Disease
Based on our findings in lupus patients and evidence that patients with RA also often die prematurely from cardiovascular disease, we thought it would be worthwhile to apply our protocol to examine the prevalence and clinical and biological features of preclinical atherosclerosis in RA.
In addition, having collected considerable data from our lupus and control cohorts, we were poised to ask a new question: What is the prevalence of atherosclerotic cardiovascular disease in RA compared with that in SLE? Although markers of disease severity in RA have been linked to an increase in overall mortality, the specific aspects of RA or its treatment that might heighten the risk of cardiovascular disease were not known.12,13
Even in the absence of traditional risk factors for atherosclerosis, women with RA had high rates of non-fatal myocardial infarction.14,15 Corticosteroids did not appear to increase the risk of cardiovascular events, while therapy with methotrexate was associated with lower all-cause mortality, largely due to a reduction in cardiovascular mortality, suggesting that some treatments of RA might mitigate cardiovascular risk.16 As was the case for SLE, however, most published work was retrospective, event rates were low, and there were no controls for the impact of conventional risk factors, treatment, and disease activity and severity.
Here again, we assessed the prevalence of atherosclerosis employing ultrasound-defined carotid artery plaque as a direct measure of and proxy for generalized atherosclerosis and as a surrogate for coronary atherosclerosis. We recruited 98 RA patients who were matched with controls and SLE patients on the basis of age (± five years), gender, and race.
More Atherosclerosis in Patients with RA
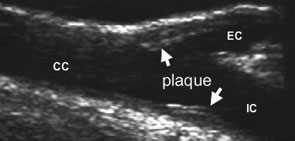
Despite a more adverse risk profile in control subjects (slightly higher blood pressure, more smokers, and lower HDL cholesterol), carotid atherosclerosis was three times more prevalent in the patient group (44% versus 15%, p<0.001).17 The presence of atherosclerosis was higher in RA patients in all decades of life; the difference was particularly striking among younger subjects. In fact, the prevalence of plaque in RA patients was comparable to that in SLE, a most dramatic and unexpected result. (See Figure 2, below.) The association between RA and atherosclerosis remained after accounting for age, cholesterol, smoking history, and blood pressure.17
Although others have found increased intimal-medial thickness in RA, intimal-medial thickness did not differ between RA patients and controls. This result is comparable to our findings in SLE patients, indicating that intimal-medial thickness does not invariably correlate with atherosclerosis, particularly in relatively young individuals with chronic inflammatory disease. Indeed, plaque is the unequivocal manifestation of atherosclerosis, and a more potent predictor of adverse cardiovascular outcomes than intimal-medial thickness.
We found that RA patients with plaque were more likely to have conventional cardiovascular risk factors (older age, higher LDL cholesterol levels and systolic blood pressure), and more severe RA (longer duration, higher Multidimensional Health Assessment Questionnaire [MDHAQ] score, and a larger number of fixed joint deformities).17
Treatment Patterns and Inflammation Markers
RA treatment was similar in both groups, with the exception of more common use of anti-tumor necrosis factor (TNF) agents in patients with atherosclerosis. Of note, the presence of plaque was not affected by the use of COX-2 inhibitors. Thus, in our studies, more vigorous treatment of SLE patients was associated with lower plaque prevalence, but in RA, the use of anti-TNF agents was associated with higher plaque prevalence.
Our patients’ initial diagnoses of RA antedated the availability of anti-TNF agents (mean duration of disease of 12 ± 10 years), which were added later to the regimens of patients with disease refractory to conventional treatment. The administration of these agents in our study may therefore represent a surrogate marker of disease activity and damage rather than an atherogenic effect of the drug. This interpretation is supported by a higher number of fulfilled RA diagnostic criteria and higher MDHAQ scores in patients who had used anti-TNF agents compared with those who had not.17
Markers of systemic inflammation have been shown to confer increased risk for cardiovascular death in RA suggesting that inflammatory mediators may play a direct role in ASCVD pathogenesis.13,18 We did not find differences in CRP, ESR, IL-6, and circulating adhesion molecules between RA patients without and with atherosclerosis but, as was the case in our lupus study, these circulating inflammatory markers were elevated compared with the normal range.

Both our studies were limited by their cross-sectional design and inherent inability to establish causality rather than document associations. Single determinations of inflammatory markers may not accurately represent concentrations over time and the cumulative burden of exposure. In addition, we were unable to precisely quantify lifetime dosages of medications to examine more fully and accurately the impact of pharmacologic therapy on the development of atherosclerosis.
Although we did not identify a biomarker for atherosclerosis in our patients, we demonstrated an excess of preclinical atherosclerosis in RA and SLE compared to a matched control group, an increase that was most striking in younger individuals. Traditional ASCVD risk factors were not primarily responsible for accelerated atherosclerosis, and corticosteroid therapy was certainly not an atherogenic factor in these patients.
Take Control of Cardiac Risk Management
The evidence for accelerated atherosclerosis in SLE and RA is compelling, yet studies show that rheumatologists are not adequately implementing preventive strategies.19,20 In our study, RA patients with plaque had a mean total cholesterol value of 226 mg/dL, but therapy with lipid-lowering agents was uncommon (approximately 5%).
Some rheumatologists argue that they are responsible for monitoring and treating only rheumatic disease and that internists or cardiologists should manage cardiovascular risk factors. My co-researchers and I believe that such a posture is not justified.
The presence of either SLE or RA constitutes a sufficiently potent risk factor for ASCVD that more aggressive goals for risk factor modification need to be adopted and vigorously pursued, goals analogous to the American Heart Association recommendations for risk reduction in diabetes mellitus.21-23 (See Table 1, above right). Furthermore, we believe that it is the rheumatologist’s responsibility to address the increased propensity to cardiovascular disease in SLE and RA patients, either directly or by advising the patient’s internist.
Treatment Goals
The practical implications of a more aggressive strategy relate to achieving lower targets for blood pressure and LDL cholesterol as recently established by the American Heart Association.21-23 The standard goal had been to maintain blood pressure <140/90 mmHg with lifestyle modification and, if necessary, pharmacological therapy, whereas <130/80 mmHg is the more aggressive stance. It must be noted that in certain clinical situations, especially among the elderly, vigorous measures to lower blood pressure carry their own risks.
The former targets for LDL cholesterol are <160 mg/dL in the setting of less than two risk factors, <130 mg/dL in the setting of at least two risk factors, and less than <100 mg/dL (or, possibly, <70 mg/dL) in the presence of clinical ASCVD or its equivalent (i.e., diabetes). We suggest, in keeping with the new recommendations, that LDL cholesterol be lowered to <100 mg/dL in SLE and RA patients, and to <70 mg/dL or lower in patients with documented ischemic events.
TABLE 1: Strategies to Reduce ASCVD in Patients with SLE and RA
- Hypertension Goal: BP <130/80 mmHg Method: ACE inhibitors
- Hyperlipidemia Goal: LDL <100 mg/dL, particularly if preclinical ASCVD is present Method: statins
- Hyperglycemia Method: maintain normal fasting plasma glucose
- Smoking cessation Method: nicotine replacement and formal cessation programs
- Weight management Goal: BMI <25 kg/m2
- Physical activity Goal: at least 30 minutes/day, three to four times/week
Because dietary changes are often inadequate to achieve these goals, pharmacological therapy is often required, with an HMG-CoA reductase inhibitor (statin) the drug of first choice. Statins are particularly attractive because they effectively lower LDL cholesterol (without lowering the HDL fraction) and also decrease levels of C-reactive protein, in patients with documented coronary artery disease.
The use of antimalarial agents in the treatment of SLE may provide an additional beneficial effect on serum lipid levels. Aspirin therapy is recommended for primary prevention for ASCVD in men; it is, as yet, of unproven benefit in women and not recommended for primary prevention in women with SLE or RA.
The use of NSAIDs is not contraindicated, but such therapy should be individualized keeping in mind the potential risks and benefits. The precise nature and magnitude of the cardiovascular risk imparted by traditional NSAIDs and COX-2 inhibitors is a subject of controversy and requires further study.
Research and Debate Will Refine Treatment
This aggressive approach is particularly important in SLE and RA patients in whom preclinical ASCVD is detected. The relative merits of the various non-invasive tests to detect preclinical ischemia or atherosclerosis and thereby identify high-risk individuals are beyond the scope of this commentary, and the economic implications of systematic non-invasive testing in SLE and RA patients must be considered before adopting a public policy of widespread screening.
Ongoing research may identify subsets of SLE and RA patients who are at heightened risk of premature atherosclerosis based on clinical identifiers. For the present, close adherence to guidelines for primary prevention of ASCVD and a lower threshold for more aggressive interventions are warranted in our SLE and RA.
Further, now that it is clear that chronic inflammation is a driving force for premature atherosclerosis, we also must be more aggressive in managing lupus and RA disease activity. We may find that the standard practice of using immunosuppressive therapy only for clinical flares does not inhibit chronic low-level inflammation that promotes atherosclerosis.
Dr. Salmon is senior scientist of the program in autoimmunity and inflammation at Hospital for Special Surgery and professor of medicine at Weill Medical College of Cornell University in New York City.
References
- Bulkley BH, Roberts WC. The heart in SLE and the changes induced in it by corticosteroid therapy. A study of 36 necropsy cases. Am J Med. 1975;53:243-264.
- Urowitz MB, Bookman AA, Koehler BE, Gordon DA, Smythe HA, Ogryzlo MA. The bimodal mortality pattern of SLE. Am J Med. 1976;60:221-225.
- Aranow C, Ginzler EM. Epidemiology of cardiovascular disease in systemic lupus erythematosus. Lupus. 2000;9:166-169.
- Manzi S, Meilahn EN, Rairie J, et al. Age-specific incidence rates of myocardial infarction and angina in women with systemic lupus erythematosus: Comparison with the Framingham study. Amer J Epidemiol. 1997;145:408-415.
- Esdaile JM, Abrahamowicz M, Grodzicky T, et al. Traditional Framingham risk factors fail to fully account for accelerated atherosclerosis in systemic lupus erythematosus. Arthritis Rheum. 2001;44:2331-2337.
- Ross R. Atherosclerosis-an inflammatory disease. N Engl J Med. 1999;340:115-126.
- Libby P. Inflammation in atherosclerosis. Nature. 2002; 420:868-874.
- Hansson GK. Inflammation, atherosclerosis, and coronary artery disease. N Engl J Med. 2005;352:1685-1695.
- Hulthe J, Wikstrand J, Emanuelsson H, et.al. Atherosclerotic changes in the carotid artery bulb as measured by B-mode ultrasound are associated with the extent of coronary atherosclerosis. Stroke. 1997;28:1189-1194.
- Belcaro G, Nicolaides AN, Laurora G, et al. Ultrasound morphology classification of the arterial wall and cardiovascular events in a 6-year follow-up study. Arterioscler Thromb Vasc Biol. 1996;16:851-856.
- Roman MJ, Shanker B-A, Davis A, et al. Prevalence and correlates of accelerated atherosclerosis in systemic lupus erythematosus. New Engl J Med. 2003;349:2399-2406.
- Wolfe F, Mitchell DM, Sibley JT, et al. The mortality of rheumatoid arthritis. Arthritis Rheum. 1994;37:481-494.
- Goodson NJ, Wiles NJ, Lunt M, Barrett EM, Silman AJ, Symmons DP. Mortality in early inflammatory polyarthritis: cardiovascular mortality is increased in seropositive patients. Arthritis Rheum. 2002;46:2010-2019.
- Solomon DH, Karlson EW, Rimm EB, et al. Cardiovascular morbidity and mortality in women diagnosed with rheumatoid arthritis. Circulation. 2003;107:1303-1307.
- del Rincón I, Williams K, Stern MP, Freeman Gl, Escalante A. High incidence of cardiovascular events in a rheumatoid arthritis cohort not explained by traditional cardiac risk factors. Arthritis Rheum. 2001;44:2737-2745.
- Choi HK, Hernán MA, Seeger JD, Robins JM, Wolfe F. Methotrexate and mortality in patients with rheumatoid arthritis: a prospective study. Lancet. 2002;359:1173-1177.
- Roman M, Moeller E, Davis A, et al. Preclinical carotid atherosclerosis in patients with rheumatoid arthritis: prevalence and associated factors. Ann Intern Med. 2006;144:249-256.
- Maradit-Kremers H, Nicola PJ, Crowson CS, Ballman KV, Gabriel SE. Cardiovascular death in rheumatoid arthritis: a population-based study. Arthritis Rheum. 2005;52:722-732.
- Al-Herz A, Ensworth S, Shojania K, Esdaile JM. Cardiovascular risk factor screening in systemic lupus erythematosus. J Rheumatol. 2003 30:493-496.
- Costenbader KH, Wright E, Liang MH, Karlson EW. Cardiac risk factor awareness and management in patients with systemic lupus erythematosus. Athritis Rheum. 2004;51:983-988.
- Smith SC, Becker D, Clark LT, et al. Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) Final Report. Circulation. 2002;106:3143-3421.
- Grundy SM, Cleeman JI, Merz CN, et al. Implications of Recent Clinical Trials for the National Cholesterol Education Program Adult Treatment Panel III Guidelines. Circulation. 2004; 110:227-239.
- Smith SC Jr, Allen J, Blair SN, et al. MD AHA/ACC Guidelines for Secondary Prevention for Patients With Coronary and Other Atherosclerotic Vascular Disease: 2006 Update. Circulation. 2006;113:2363-2372.

