 In medicine, as in advertising, pictures can be worth a thousand words. From arthritis to vasculitis, imaging studies have been variably employed to aid in the diagnosis, treatment, risk stratification and prognostication of patients with rheumatic and musculoskeletal disorders. The same holds true with the idiopathic inflammatory myopathies (IIM), in which the clinical utility is high, despite the absence of imaging as a diagnostic variable in the latest European League Against Rheumatism (EULAR)/ACR classification criteria.1
In medicine, as in advertising, pictures can be worth a thousand words. From arthritis to vasculitis, imaging studies have been variably employed to aid in the diagnosis, treatment, risk stratification and prognostication of patients with rheumatic and musculoskeletal disorders. The same holds true with the idiopathic inflammatory myopathies (IIM), in which the clinical utility is high, despite the absence of imaging as a diagnostic variable in the latest European League Against Rheumatism (EULAR)/ACR classification criteria.1
These criteria—intended for research classification purposes and not diagnosis—took into account the availability of specific tests, and magnetic resonance imaging (MRI), the gold standard imaging modality for muscle, is not available in all regions. Nevertheless, imaging remains of particular importance in IIM because it provides an assessment of structural abnormalities in muscle tissue, which can help confirm the presence of disease, delineate the type and pattern of its involvement and, ultimately, help guide treatment.
MRI: Myositis Routine Imaging?
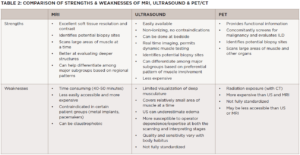
MRI provides excellent soft tissue resolution and contrast, allowing for the evaluation of the extent and distribution of edema, fatty/fibrous replacement and atrophy, and is the main workhorse when it comes to muscle imaging. It is useful for diagnostic purposes to confirm muscle involvement in a patient with suspected disease and can pinpoint potentially high-yield sites for muscle biopsy, albeit not in real time. In some instances, it can help differentiate among the IIM subgroups based on muscles involved and distinguish them from common mimics (see Table 1).
The MRI myositis protocol involves T1-weighted imaging (T1W) and fluid-sensitive T2W with fat suppression or short-tau inversion recovery (STIR) sequence in the axial and coronal planes.2,3 Gadolinium contrast is not required unless fasciitis, focal myositis or a cystic or solid mass lesion are of primary concern.4 In IIM-affected muscle, T2W and STIR hyperintensities depict edema and increased water content characteristic of active muscle inflammation or necrosis in the acute phase (see Figure 1B). T1W hyperintensities delineate fatty replacement which is a marker of damage and chronicity (see Figure 1A). Calcinosis can be detected on all sequences as signal voids (see Table 1).
A whole-body MRI would be ideal in that it can yield precise patterns of preferential muscle involvement or sparing, making it possible to detect disease-specific muscle signatures or fingerprints. Clinically, however, it is time consuming and cost inefficient. Thus, an MRI of the thigh or of the upper extremity is typically employed, and generally sufficient, for the evaluation of IIMs. In the same vein, while higher magnetic strengths may yield crisper images, the 1.5 Tesla (1.5T) MRI does not appear to fare worse than the 3.0T for the purposes of MSK imaging.5
Even after the diagnosis of IIM has been established, MRI remains useful to monitor response to treatment, qualitatively measure disease activity, and direct future management. This is particularly important when discordance exists between the patients’ symptoms and providers’ assessments and when routine serologic markers of muscle breakdown fail to correlate with clinical findings. Active disease requiring escalation or modification of therapy is indicated by more pronounced muscle edema, whereas response to therapy appears as resolution or reduction of edema (normalization of intensity values or “return to isointensity” in the above sequences). Notably, a relationship between the degree of STIR hyperintensity and the abundance of pre-treatment inflammatory infiltrates on muscle biopsy has been shown in several studies.4,6
On the other hand, intensification of the largely irreversible markers of chronicity and damage, such as fatty replacement and atrophy (increased signal intensity and decreased muscle mass, respectively, on T1W), warrant reevaluation of the drug regimen to balance out the need to prevent both disease progression and unnecessary exposure to potentially harmful medications. Quantitative MRI studies have similarly demonstrated a modest correlation between the extent of fatty infiltration noted on MRI and that seen on muscle histopathology.7,8
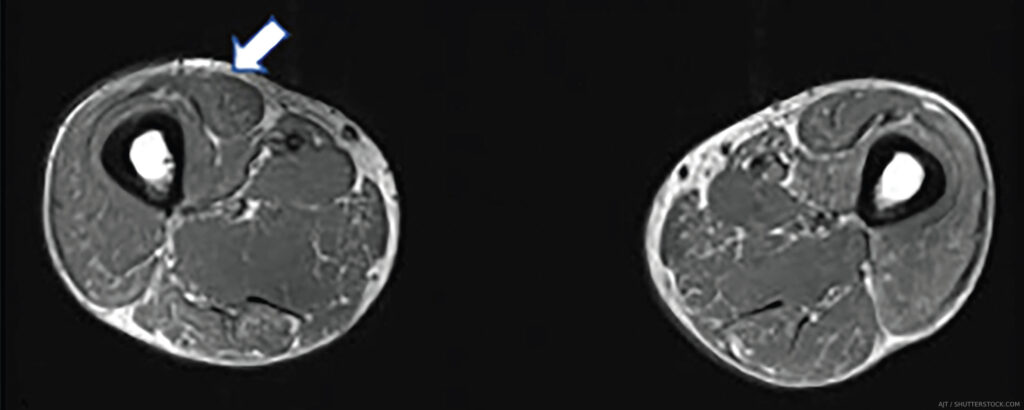
Thus, the capacity of MRI findings to correlate with response to therapy underscores its value in guiding subsequent decision making. With its versatility, granularity and increasing ubiquity, it’s easy to see why MRI is the go-to muscle imaging modality (see Table 2).
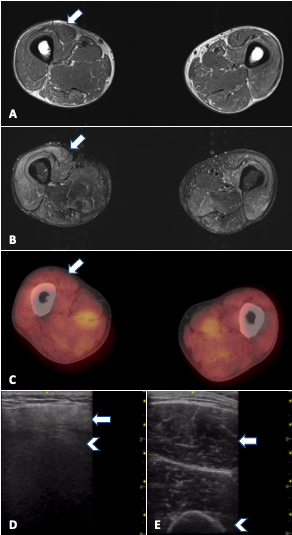
FIGURE 1. MRI, PET/CT and ultrasound in a 33-year-old man with refractory PM-Scl dermatomyositis. Arrows: rectus femoris; arrowheads: humerus. A) MRI of the thighs (T1W) showing no significant fatty replacement. B) MRI of thighs (STIR) with mild to moderate muscle edema (increased signal) mainly in anterior compartment. C) PET/CT showing symmetric bilateral increased FDG uptake in scattered muscles, especially pelvic adductors, D) increased muscle echointensity in the rectus femoris with attenuation (loss of bone echo of underlying femur) and atrophy in comparison with E) normal rectus femoris in age-matched control.
Ultrasound
Ultrasound is undeniable proof that oldies can still be goodies. With the advent of higher frequency probes, imaging resolution for soft tissue is now far higher than MRI and offers a close-up view of the muscle, which has its own advantages. Ultrasound also has more widespread availability, portability, cost efficiency, and real-time imaging capability, with the latter rendering it a useful tool for optimizing muscle biopsy site selection as well as for detecting abnormal muscle movements (see Table 2). However, ultrasound is heavily operator dependent, and knowledge of both proper image acquisition and image interpretation for muscle is needed.
On ultrasound, normal muscle is relatively anechoic (black) or hypoechoic (gray) and interspersed with hyperechoic (white) perimysial septa, which gives it a starry sky appearance on cross-section (see Figure 1E). Longitudinally, the parallel orientation of muscle fibers is appreciated. Myopathic muscle is marked by an increase in muscle echointensity, mild in acute muscle edema, with a more marked increase with fatty fibrous replacement. Changes in muscle architecture are also seen, as well as loss of visualization of structures deeper to the muscle (attenuation) when pathology is severe (see Figure 1D).
In cases of acute edema, a see-through echogenicity is described, in which there is no attenuation of ultrasound waves despite an increase in muscle echointensity.9 Notably, acute myopathic changes can be subtle, and detecting edema may be inconsistent on ultrasound. Ultrasound is very useful, however, to pick up chronic changes given its sensitivity for fat/fibrosis and atrophy. Unlike MRI where different sequences can delineate either edema or fatty replacement, ultrasound shows a combination of all pathology in one B mode image. In terms of vascularity, a few studies suggest that higher Doppler scores are seen with acute myositis.10,11 Although the use of Power Doppler is a must in inflamed joints, a need exists for better standardization for muscle because perfusion is affected by multiple factors, including activity and muscle contraction.
Ultrasound has been gaining traction for diagnostic purposes especially in chronic myositis, such as inclusion body myositis (IBM) in which the specificity of the affected muscles can help distinguish it from mimics.12 It may find more use as a follow-up tool in treatable cases of myositis by showing the successful resolution of increased muscle echogenicity or the development of fatty, fibrous replacement. The routine use of such modalities as elastography, evaluating tissue stiffness, may provide further information about muscle quality in the future. Despite the formidability of MRI for myositis, ultrasound is a viable alternative in the hands of experienced operators.
PET
On the other end of the innovation spectrum is metabolic imaging—the nuclear imaging technique of positron emission tomography (PET), which is most often combined with computed tomography (CT) or MRI to provide concurrent metabolic and anatomic information of tissues. The most commonly used tracer of 18F-FDG picks up an increase in glycolysis that occurs in the setting of inflammation.
Other tracers—such as 15O-water, which detects blood flow, and 11C-L-methylmethionine, which targets amino acid transport—have been used to evaluate skeletal muscle; however, their considerably shorter half-lives compared with 18F-FDG translate to shorter—and thus less optimal—post-injection imaging windows.13,14 For IBM specifically, the amyloid markers 11C-PIB[97] and 18F-florbetapir and the tau marker 18F-THK5317 may have value in differentiating IBM from other IIMs.15-17
It is important to note that not all instances of MRI-detected muscle edema in IIM correlate with an increase in 18F-FDG activity. The detection of increased 18F-FDG activity may provide another layer of information regarding the disease process (see Figure 1C, right). Additionally, its utility in screening for malignancy in newly diagnosed or refractory cases of myositis is being invoked, as well as its ability to follow interstitial lung disease.18,19
PET imaging may be uniquely advantageous to the subset of patients considered at high risk for malignancy and interstitial lung disease based on autoantibody status and other predictors (see Table 2). PET/MR, which offers higher soft tissue contrast than PET/CT without the ionizing radiation, may yet emerge as a mainstream modality in IIM imaging.
Conclusion
A thorough clinical evaluation aided by serologic and histopathologic findings remain at the forefront of the assessment and management of IIMs; however, there is plenty of room to harness the power of imaging studies, such MRI, ultrasound and multimodal PET to better characterize disease parameters across the entire clinical trajectory. While each modality can certainly hold its own in the myositis imaging space, they can also be used either simultaneously or sequentially to paint the most accurate picture of a patient’s condition over time.
Capitalizing on the major strengths of each imaging approach—the clarity for muscle edema vs. fat/fibrosis with MRI, the real-time and dynamic assessment with ultrasound, and the physiologic information of PET—can provide clinicians with the images that best capture what is needed to positively influence decision making and overall outcomes.
With the broad shift toward less invasive, more patient-centric approaches in all aspects of patient care, these pictures will certainly be worth far more than a thousand words in the not-too-distant future.
 Rochelle L. Castillo, MD, MS, is a clinical instructor in the Division of Rheumatology at NYU Grossman School of Medicine, New York, where she also completed her rheumatology fellowship. She is a clinician-investigator with a specific interest in conditions that bridge the rheumatology-dermatology interface, such as psoriatic disease and dermatomyositis.
Rochelle L. Castillo, MD, MS, is a clinical instructor in the Division of Rheumatology at NYU Grossman School of Medicine, New York, where she also completed her rheumatology fellowship. She is a clinician-investigator with a specific interest in conditions that bridge the rheumatology-dermatology interface, such as psoriatic disease and dermatomyositis.
 Andro Licaros, MD, is a clinical fellow in the Cancer Imaging Program at Dana-Farber Cancer Institute/Harvard Cancer Center, Boston. His research work focuses on cancer imaging, and quality and safety improvements in radiology leveraging machine learning and artificial intelligence toward patient-centric outcomes.
Andro Licaros, MD, is a clinical fellow in the Cancer Imaging Program at Dana-Farber Cancer Institute/Harvard Cancer Center, Boston. His research work focuses on cancer imaging, and quality and safety improvements in radiology leveraging machine learning and artificial intelligence toward patient-centric outcomes.
 Jemima Albayda, MD, is an assistant professor of medicine in the Division of Rheumatology at the Johns Hopkins University School of Medicine, Baltimore. She is the director of the Rheumatology Fellowship Program, as well as the Musculoskeletal Ultrasound and Injection Clinic. Her clinical and research focus is in the inflammatory muscle diseases, musculoskeletal ultrasound and arthritis.
Jemima Albayda, MD, is an assistant professor of medicine in the Division of Rheumatology at the Johns Hopkins University School of Medicine, Baltimore. She is the director of the Rheumatology Fellowship Program, as well as the Musculoskeletal Ultrasound and Injection Clinic. Her clinical and research focus is in the inflammatory muscle diseases, musculoskeletal ultrasound and arthritis.
References
- Lundberg IE, Tjärnlund A, Bottai M, et al. 2017 European League Against Rheumatism/American College of Rheumatology classification criteria for adult and juvenile idiopathic inflammatory myopathies and their major subgroups. Ann Rheum Dis. 2017 Dec;76(12):1955–1964.
- Costa AF, Di Primio GA, Schweitzer ME. Magnetic resonance imaging of muscle disease: A pattern-based approach. Muscle Nerve. 2012 Oct;46(4):465–481.
- Tomasová Studýnková J, Charvát F, Jarošová K, Vencovský J. The role of MRI in the assessment of polymyositis and dermatomyositis. Rheumatology (Oxford). 2007
Jul;46(7):1174–1179.
- Malartre S, Bachasson D, Mercy G, et al. MRI and muscle imaging for idiopathic inflammatory myopathies. Brain Pathol. 2021 May;31(3):e12954.
- Smith AC, Parrish TB, Abbott R, et al. Muscle-fat MRI: 1.5 Tesla and 3.0 Tesla versus histology. Muscle Nerve. 2014 Aug;50(2):170–176.
- Malartre S, Bachasson D, Mercy G, et al. ibid.
- Güttsches A-K, Rehmann R, Schreiner A, et al. Quantitative muscle-MRI correlates with histopathology in skeletal muscle biopsies. J Neuromuscul Dis. 2021;8(4):669–678.
- Lassche S, Küsters B, Heerschap A, et al. Correlation between quantitative MRI and muscle histopathology in muscle biopsies from healthy controls and patients with IBM, FSHD and OPMD. J Neuromuscul Dis. 2020;7(4):495–504.
- Albayda J, van Alfen N. Diagnostic value of muscle ultrasound for myopathies and myositis. Curr Rheumatol Rep. 2020 Sep 28;22(11):82.
- Conticini E, Falsetti P, Al Khayyat SG, et al. A novel grey scale and Power Doppler ultrasonographic score for idiopathic inflammatory myopathies: Siena Myositis Ultrasound Grading Scale. Rheumatology (Oxford). 2021 Dec 24;61(1):185–194.
- Meng C, Adler R, Peterson M, Kagen L. Combined use of power Doppler and grayscale sonography: A new technique for the assessment of inflammatory myopathy. J Rheumatol. 2001 Jun;28(6):1271–1282.
- Leeuwenberg KE, Alfen N van, Christopher-Stine L, et al. Ultrasound can differentiate inclusion body myositis from disease mimics. Muscle Nerve. 2020 Jun;61(6):783–788.
- Chaudhari AJ, Raynor WY, Gholamrezanezhad A, et al. Total-body PET imaging of musculoskeletal disorders. PET Clin. 2021 Jan;16(1):99–117.
- Wahl RL, Herman JM, Ford E. The promise and pitfalls of positron emission tomography and single-photon emission computed tomography molecular imaging-guided radiation therapy. Semin Radiat Oncol. 2011 Apr;21(2):88–100.
- Noto Y-I, Kondo M, Tsuji Y, et al. Diagnostic value of muscle [11C]PIB-PET in inclusion body myositis. Front Neurol. 2020 Jan 17;10:1386.
- Lilleker JB, Hodgson R, Roberts M, et al. [18F]Florbetapir positron emission tomography: Identification of muscle amyloid in inclusion body myositis and differentiation from polymyositis. Ann Rheum Dis. 2019 May;78(5):657–662.
- Zhang Y, Li K, Pu C, Dang H, Liu J, Shi Q. A novel application of tau PET in the diagnosis of sporadic inclusion body myositis: A case report. Medicine (Baltimore). 2020 Jul 31;99(31):e21524.
- Kundrick A, Kirby J, Ba D, et al. Positron emission tomography costs less to patients than conventional screening for malignancy in dermatomyositis. Semin Arthritis Rheum. 2019 Aug;49(1):140–144.
- Liang J, Cao H, Liu Y, et al. The lungs were on fire: A pilot study of 18F-FDG PET/CT in idiopathic-inflammatory-myopathy-related interstitial lung disease. Arthritis Res Ther. 2021 Jul;23(1):198.