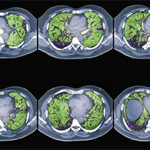
With respect to selecting which patients should be treated for CTD-ILD, Dr. Highland explained that patients with ≥20% involvement on high-resolution CT and clinical disease progression as evidenced by 10% drop in FVC and/or 5–9% FVC decline with a 15% diffusing capacity of the lungs for carbon monoxide (DLCO) decline can be considered candidates for therapy.
Risk Factors for Disease Progression
Within each connective tissue disease, it is also helpful to identify risk factors for progression of lung disease, Dr. Highland noted. In scleroderma, for example, autoantibodies directed against topoisomerase I, diffuse cutaneous disease and African American race are among the risk factors associated with ILD progression.4
Risk factors for ILD in patients with rheumatoid arthritis include tobacco smoking, male sex, the presence of rheumatoid factor and anti-cyclic citrullinated peptide antibodies in high titer, and onset of rheumatoid arthritis after age 50.
In patients with idiopathic inflammatory myopathies, anti-synthetase antibodies are clearly associated with ILD, and anti-melanoma differentiation-associated protein-5 (anti-MDA5) antibodies can be seen in patients with particularly severe and rapidly progressive ILD.5
SENSCIS Trial
The SENSCIS trial is a landmark study in the world of CTD-ILD. In this study, Distler et al. randomized 576 patients with ILD associated with systemic sclerosis to receive either nintedanib, a tyrosine kinase inhibitor, at a dose of 150 mg by mouth twice daily, or placebo. Fifty-two percent of the patients had diffuse cutaneous disease, and 48% were receiving mycophenolate mofetil at baseline.
The authors found the adjusted annual rate of change in FVC was −52.4 mL per year in the patients receiving nintedanib and −93.3 mL per year in the group receiving placebo (95% confidence interval 2.9−79, P=0.04); no clinical benefit in other manifestations of scleroderma was seen in the nintedanib group, and the primary side effect of this medication was diarrhea (75.7% of patients receiving nintedanib vs. 31.6% in the placebo group).6
Although the study was not designed to show a difference between patients who were or were not receiving mycophenolate mofetil as background therapy, a subgroup analysis showed that patients receiving mycophenolate at baseline and randomized to nintedanib had the smallest mean absolute change from baseline in FVC of all groups.7
A separate study of nintedanib in progressive fibrosing interstitial lung disease, in which about 25% of patients had autoimmune ILDs, helped provide further evidence of the potential benefit of this treatment for some patients.8 This led to nintedanib, in March 2020, becoming the first treatment approved by the U.S. Food & Drug Administration for the treatment of chronic fibrosing ILD with a progressive phenotype.