 ACR Convergence 2021—The past year has been an exciting time for researchers and clinicians focused on the topic of connective tissue disease-associated interstitial lung disease (CTD-ILD), with advances in our understanding of these disorders and newly approved medications for their treatment. At the ACR Convergence 2021 annual Review Course, Kristin Highland, MD, MSCR, director of the Rheumatic Lung Disease Program at the Cleveland Clinic, provided an exceptional overview of this topic and future developments to expect in the field.
ACR Convergence 2021—The past year has been an exciting time for researchers and clinicians focused on the topic of connective tissue disease-associated interstitial lung disease (CTD-ILD), with advances in our understanding of these disorders and newly approved medications for their treatment. At the ACR Convergence 2021 annual Review Course, Kristin Highland, MD, MSCR, director of the Rheumatic Lung Disease Program at the Cleveland Clinic, provided an exceptional overview of this topic and future developments to expect in the field.
Significant Source of Morbidity & Mortality

Dr. Highland
Dr. Highland began by noting that CTD-ILD can be prevalent in certain rheumatic diseases and can represent a significant source of morbidity and mortality. In scleroderma, autopsy studies have demonstrated the presence of ILD in 75–95% of patients and, although scleroderma renal crisis was the leading cause of death in patients in the early 1970s, pulmonary fibrosis became the leading cause of mortality in patients with scleroderma by the early 2000s.1
In patients with rheumatoid arthritis, the prevalence of ILD is estimated to be 5–58%, with a 7.7% lifetime risk of developing this condition, which can precede articular disease in up to 20% of patients and is responsible for 10–20% of deaths.2,3
Radiographic Pattern of Disease
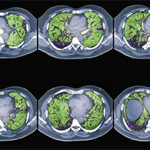
Once ILD has been identified in a patient, Dr. Highland explained that it is important to understand the radiographic pattern of disease to predict prognosis. Nonspecific interstitial pneumonia combines reticulations and ground glass opacities that tend to be peripheral with an apical to basilar gradient. This is the most common pattern of ILD seen in connective tissue diseases. The presence of ground glass opacities implies inflammatory changes that are potentially reversible. Such findings may also be seen with organizing pneumonia, particularly in patients with rheumatoid arthritis, lupus and idiopathic inflammatory myopathies.
In contrast, lymphocytic interstitial pneumonitis, most often seen in patients with Sjögren’s syndrome, and interstitial pneumonia, which can be seen in patients with idiopathic pulmonary fibrosis and rheumatoid arthritis, are fibrotic conditions that are not typically reversible with immunosuppression.
Dr. Highland showed that by combining knowledge of the pattern of ILD seen on high-resolution computed tomography (CT) imaging, the forced vital capacity (FVC) as determined by pulmonary function testing and the histopathologic pattern of disease seen on lung biopsy, patients can be grouped into subsets of CTD-ILD and survival rates can be predicted with reasonable accuracy.
With respect to selecting which patients should be treated for CTD-ILD, Dr. Highland explained that patients with ≥20% involvement on high-resolution CT and clinical disease progression as evidenced by 10% drop in FVC and/or 5–9% FVC decline with a 15% diffusing capacity of the lungs for carbon monoxide (DLCO) decline can be considered candidates for therapy.
Risk Factors for Disease Progression
Within each connective tissue disease, it is also helpful to identify risk factors for progression of lung disease, Dr. Highland noted. In scleroderma, for example, autoantibodies directed against topoisomerase I, diffuse cutaneous disease and African American race are among the risk factors associated with ILD progression.4
Risk factors for ILD in patients with rheumatoid arthritis include tobacco smoking, male sex, the presence of rheumatoid factor and anti-cyclic citrullinated peptide antibodies in high titer, and onset of rheumatoid arthritis after age 50.
In patients with idiopathic inflammatory myopathies, anti-synthetase antibodies are clearly associated with ILD, and anti-melanoma differentiation-associated protein-5 (anti-MDA5) antibodies can be seen in patients with particularly severe and rapidly progressive ILD.5
SENSCIS Trial
The SENSCIS trial is a landmark study in the world of CTD-ILD. In this study, Distler et al. randomized 576 patients with ILD associated with systemic sclerosis to receive either nintedanib, a tyrosine kinase inhibitor, at a dose of 150 mg by mouth twice daily, or placebo. Fifty-two percent of the patients had diffuse cutaneous disease, and 48% were receiving mycophenolate mofetil at baseline.
The authors found the adjusted annual rate of change in FVC was −52.4 mL per year in the patients receiving nintedanib and −93.3 mL per year in the group receiving placebo (95% confidence interval 2.9−79, P=0.04); no clinical benefit in other manifestations of scleroderma was seen in the nintedanib group, and the primary side effect of this medication was diarrhea (75.7% of patients receiving nintedanib vs. 31.6% in the placebo group).6
Although the study was not designed to show a difference between patients who were or were not receiving mycophenolate mofetil as background therapy, a subgroup analysis showed that patients receiving mycophenolate at baseline and randomized to nintedanib had the smallest mean absolute change from baseline in FVC of all groups.7
A separate study of nintedanib in progressive fibrosing interstitial lung disease, in which about 25% of patients had autoimmune ILDs, helped provide further evidence of the potential benefit of this treatment for some patients.8 This led to nintedanib, in March 2020, becoming the first treatment approved by the U.S. Food & Drug Administration for the treatment of chronic fibrosing ILD with a progressive phenotype.
In Sum
Dr. Highland’s remarks illustrate why there is a great deal of optimism for the future treatment of CTD-ILD.
Jason Liebowitz, MD, completed his fellowship in rheumatology at Johns Hopkins University, Baltimore, where he also earned his medical degree. He is currently in practice with Skylands Medical Group, N.J.
References
- Steen VD, Medsger TA. Changes in causes of death in systemic sclerosis, 1972–2002. Ann Rheum Dis. 2007 Jul;66(7):940–944.
- Doyle TJ, Patel AS, Hatabu, H, et al. Detection of rheumatoid arthritis-interstitial lung disease is enhanced by serum biomarkers. Am J Respir Crit Care Med. 2015 Jun 15;191(12):1403–
- Bongartz T, Nannini C, Medina-Velasquez YF, et al. Incidence and mortality of interstitial lung disease in rheumatoid arthritis: A population-based study. Arthritis Rheum. 2010 Jun;62(6):1583–15
- Perelas A, Silver RM, Arrossi AV, et al. Systemic sclerosis-associated interstitial lung disease. Lancet Respir Med. 2020 Mar;8(3):304–
- Nombel A, Fabien, N, Coutant F. Dermatomyositis with anti-MDA5 antibodies: Bioclinical features, pathogenesis and emerging therapies. Front Immunol. 2021 Oct 20;12:773352.
- Distler O, Highland KB, Gahlemann M, et al. Nintedanib for systemic sclerosis-associated interstitial lung disease. N Engl J Med. 2019 Jun 27;380(26):2518–
- Highland KB, Distler O, Kuwana M, et al. Efficacy and safety of nintedanib in patients with systemic sclerosis-associated interstitial lung disease treated with mycophenolate: A subgroup analysis of the SENSCIS trial. Lancet Respir Med. 2021 Jan;9(1):96–
- Flaherty KR, Wells AU, Cottin V, et al. Nintedanib in progressive fibrosing interstitial lung diseases. N Engl J Med. 2019 Oct 31;381(18):1718–1727.