NEW YORK/HELSINKI (Reuters)—Amneal Pharmaceuticals could soon run out of the raw ingredients to make more of the anti-malarial drug hydroxychloroquine (HCQ) that has been touted as a potential treatment for COVID-19 because Finland is keeping the drug for domestic use, according to the generic drugmaker’s chief executives.
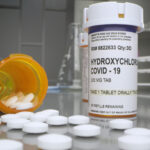
Amneal has committed to producing about 20 million HCQ tablets by mid-April, but will face challenges making any more after that because of difficulties acquiring active pharmaceutical ingredients from its supplier in Finland, co-CEOs Chirag and Chintu Patel said in an interview.
“As the demand has increased all across the globe… the Finnish government has put out an emergency order to prioritize their domestic use for local needs,” co-CEO Chintu Patel said in an interview on Tuesday.
HCQ is a decades-old drug promoted by many, including U.S. President Donald Trump, as a potential weapon against COVID-19. It has become a standard of care in areas of the U.S. hit hard by the pandemic, even though doctors prescribing it have no idea whether it works.
Demand for the drug has soared worldwide and some countries, like India, have placed restrictions on its export. Amneal currently manufactures HCQ in India and the brothers said the company was working with India to provide an exception to ship its finished product to the U.S.
Finland has not yet issued any bans or restrictions on drug exports, according to the Finnish medicines agency Fimea.
But even under normal circumstances, Finnish law requires pharmaceutical companies to commit themselves to fulfilling national needs first. Because the demand for HCQ and its active agents has surged in Finland, Finnish makers have less to export, Fimea said.
“The increased national need may impact the schedules of export deliveries, even if no export restrictions have been issued by the government,” Fimea Director Johanna Nystedt said in an email. “Surely every country hopes to obtain more hydroxychloroquine at the moment and therefore its makers’ order books are certainly full.”
The Finnish government, on the basis of the state of emergency it has issued, is preparing an amendment which if adopted would allow restricting exports of certain medicines.
Orion Corp. and its subsidiary Fermion are the two Finnish companies that make hydroxychloroquine and its raw materials. Fermion is Amneal’s supplier, according to a source familiar with the matter.
Orion said in a statement that securing its own raw materials from abroad is challenging and it would be pleased if it could deliver more.
“Finnish authorities have far-reaching rights to intervene in the operations of the actors in our field of business. In the exceptional situation Finland’s needs have priority,” Orion said. “Demand for the agent in question (API) is heavy around the world at the moment but unfortunately we cannot respond to the demand commensurately.”
Amneal said it is working to source the raw materials elsewhere, and is trying to get its Brookhaven, NY, manufacturing site FDA-approved to make more products, including HCQ. Amneal has said it is donating more than 6.5 million of the tablets to New York, Texas, Louisiana and hospitals across the country.
Chintu Patel said he believes the supply issues will ease up in two or three months.
“There are multiple suppliers, but worldwide demand, so people are allocating their inventory and we are in the process of qualifying more than one supplier so we can do what we can to help in this crisis situation,” he said.