ORLANDO—Despite the COVID-19 pandemic, the past two years have been exciting for rheumatology providers and patients. We’ve seen the U.S. Food & Drug Administration (FDA) approve new therapies and expand indications for established drugs. At the 2022 ACR Education Exchange, Jeffrey Curtis, MD, MS, MPH, Marguerite Jones Harbert-Gene Ball Endowed professor of medicine, Division of Clinical Immunology & Rheumatology, University of Alabama at Birmingham, provided an evidence-based update on new drugs on the block.
ORLANDO—Despite the COVID-19 pandemic, the past two years have been exciting for rheumatology providers and patients. We’ve seen the U.S. Food & Drug Administration (FDA) approve new therapies and expand indications for established drugs. At the 2022 ACR Education Exchange, Jeffrey Curtis, MD, MS, MPH, Marguerite Jones Harbert-Gene Ball Endowed professor of medicine, Division of Clinical Immunology & Rheumatology, University of Alabama at Birmingham, provided an evidence-based update on new drugs on the block.
New Drugs & Indications
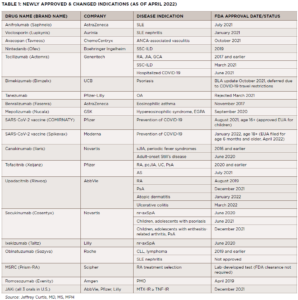
Dr. Curtis began his presentation with an outline of newly FDA-approved therapies (see Table 1). In the systemic lupus erythematosus (SLE) world, we saw the FDA approval of anifrolumab and voclosporin. For anti-neutrophil cytoplasmic antibody-associated vasculitis (AAV), we got avacopan. For systemic sclerosis-associated interstitial lung disease (SSc-ILD), we now have nintedanib and tocilizumab. FDA approval of bimekizumab for psoriasis was delayed due to COVID‑19 travel restrictions, but—given the impressive data accumulated for bimekizumab—approval is expected soon. For postmenopausal osteoporosis, there’s romosozumab. And last but not least, a new laboratory test is now available to assist with drug selection for patients with rheumatoid arthritis (RA) for whom methotrexate monotherapy has proved ineffective.

Dr. Jeffrey Curtis
When it comes to expanded indications, canakinumab is now FDA approved for adult-onset Still’s disease, tofacitinib for ankylosing spondylitis, upadacitinib for psoriatic arthritis and both secukinumab and ixekizumab for non-radiographic axial spondyloarthritis (nr-axSpA).
“As you may be aware, nr-axSpA was a controversial diagnosis, and whether the FDA would even accept this [disease] as a real thing was a concern. It’s great to have more options,” Dr. Curtis said.
Rejected Therapies
With these successes also came some failures. In March 2021, the FDA rejected tanezumab, a monoclonal antibody targeting nerve growth factor (NGF), which was developed for the treatment of osteoarthritis (OA).
“This [agent has] a promising new mechanism of action that could potentially treat OA,” said Dr. Curtis. “Despite the fact that this drug, like other NGF inhibitors, seemed to alleviate pain, a small percentage of patients studied had rapidly progressive OA or even more severe complications, [such as] osteonecrosis or tibial plateau fractures. This [FDA rejection] might be an ill harbinger for things to come for [NGF inhibitors]. We’d all like to see something new to treat OA, but this might not be it—at least in the FDA’s mind given safety concerns.”
Anifrolumab
Anifrolumab is a type I interferon (IFN) receptor antagonist approved to treat adult patients with moderate to severe SLE who are receiving standard therapy.1 Type I IFN is a known pathogenic mediator in SLE, and cell signaling by type I IFNs results in IFN-stimulated gene transcription (also known as the IFN gene signature). Some SLE patients have high IFN gene signatures, and some have low IFN gene signatures. Anifrolumab blocks the receptor that leads to IFN-stimulated gene transcription.
Dr. Curtis highlighted a curiosity of the trial that stood out to him. “Remember what this drug does: It’s a monoclonal antibody to type I IFN receptor sub-unit 1,” he said. “So wouldn’t you expect the British Isles Lupus Activity Group [BILAG] Based Composite Lupus Assessment [BICLA] response in people with a high IFN gene signature to be even better than for [those with a low IFN gene signature]? Well, it wasn’t. I find it curious that what you’d think could be a stratifying biomarker in fact wasn’t stratifying. The response rate was just about as good for people without the signature.”
He also pointed out the increased risk of herpes zoster with anifrolumab and stressed the importance of vaccination.
“The rate of herpes zoster was quite high [7.2%], but you expect that in any drug that meaningfully affects the IFN pathway,” Dr. Curtis said. “So in fact, this rate of zoster would be pretty comparable to a patient with RA on a Janus kinase inhibitor and 10 mg of prednisone daily—or maybe even a bit more. Zoster vaccination will be quite critical for the people you’re putting on this [treatment].”
When asked which type of SLE patient he would prescribe anifrolumab for, Dr. Curtis said, “Patient selection is obviously critical. Remember, this wasn’t a drug targeted for severe lupus nephritis or neuro-psychiatric SLE, and the effect on joint disease wasn’t overwhelming [but the trial wasn’t powered for that and not all patients had joint disease]. I think typical SLE manifestations with the exclusion of those three is probably where I would position it.”
Voclosporin
Voclosporin is a calcineurin inhibitor (CNI) now approved in combination with a regimen of background immunosuppressive therapy for the treatment of adult patients with active lupus nephritis. Unlike older CNIs (e.g., tacrolimus), voclosporin doesn’t require drug level monitoring.
“[Trial] inclusion criteria are important when we’re thinking about when to use this drug,” said Dr. Curtis.
Patients studied had lupus nephritis class III, IV or V confirmed with renal biopsy within the last two years, relatively preserved renal function, with an estimated glomerular filtration rate (eGFR) greater than 45 mL per minute, and urine protein-creatinine ratio of 1.5 mL/mg or more (e.g., 2 mL/mg for those with pure lupus nephritis class V) by first morning urine void.2
Voclosporin was added to 1,000 mg of mycophenolate mofetil (MMF) given twice daily with a rapid prednisone taper from 20–25 mg daily, to 2.5 mg daily over 16 weeks. The primary outcome was complete renal response, which was 41% in the voclosporin arm and 23% in placebo “despite a really rapid and aggressive prednisone taper.”
If eGFR declines after starting voclosporin, it may be hard to determine if the decline is drug-related or due to worsening lupus nephritis itself. In this case, Dr. Curtis said, “I think the timing is helpful here. If the decline is related to the drug’s mechanism of action, the decline should be early—in the first month or two. So bring people back and remeasure blood pressure and eGFR. As long as there aren’t big changes, I would be reassured.”
Nintedanib & Tocilizumab for SSc-ILD
Next, Dr. Curtis turned our attention to nintedanib and tocilizumab for SSc-ILD, both of which have been approved for slowing the rate of decline in pulmonary function in adult patients with SSc-ILD.
“The annual rate of decline in forced vital capacity [FVC] as a percent was 1.4% in the nintedanib group and 2.6% in placebo,” he said. “You might think these [findings] are pretty tiny changes over the course of the year, but multiplied by 10 years [the changes are] more impressive.”
As for tocilizumab, the primary trial end point for improvement in skin fibrosis was not met. However, secondary end point data for FVC indicated that tocilizumab may preserve lung function in this population, prompting a follow-up trial that demonstrated FVC percent predicted was preserved with tocilizumab over 48 weeks.3,4
Dr. Curtis pointed out an interesting difference in the comparator arms of these two trials, acknowledging that “it’s hard to compare trials [in an] apples to apples [fashion].” In both trials, he said, the shapes of the lung function curves were similar in terms of percent predicted change from baseline FVC on either drug. However, in the tocilizumab trial, the placebo group had a more appreciable decrease of 5–6%, as opposed to the 2.6% seen in the nintedanib trial placebo group.
‘As you may be aware, nr-axSpA was a controversial diagnosis, and whether the FDA would even accept this [disease] as a real thing was a concern. It’s great to have more options.’
—Dr. Curtis
Dr. Curtis thought this [finding] was related to the populations studied in each trial, who were different. “[In the tocilizumab trial], these were people with earlier disease that was more aggressive and progressing more quickly,” he said. “They were recruited on the basis of skin disease, but we’ve known for a while that more progressive skin and lung disease are related. The drug clearly preserved lung function, even in patients who didn’t have SSc-ILD—although the impact was more profound in those who did. The point is that if your patient has early, rapidly progressive SSc, interleukin [IL] 6 therapy may be a very promising approach to help preserve lung function.”
Bimekizumab
Bimekizumab is a promising new IL-17A and IL-17F inhibitor for patients with psoriasis. It has not yet been FDA approved due to pandemic delays, but the outlook is good given the impressive data in a 2021 New England Journal of Medicine trial.5
For bimekizumab, the Psoriasis Area and Severity Index (PASI) 90 response exceeded 90%, compared with adalimumab, which had a PASI 90 of only 40–50%. The PASI 100 also favored bimekizumab, with 70% of participants achieving completely clear skin.
“The thing I love as a clinician about the PASI 100 is that it means you have no skin disease,” Dr. Curtis said. “So the beauty of telling a patient that there is a two-thirds to three-quarters likelihood that this drug will completely get rid of your psoriasis is very powerful.”
Regarding safety, Dr. Curtis pointed out the increased incidence of oral candidiasis in patients receiving bimekizumab, which was not appreciated with adalimumab. “About 10–15% of these patients developed thrush, which is what might be expected based on what IL-17 does. But I would just tell patients to be prepared for this. These patients didn’t get deep fungal infections.”
Data specific to psoriatic arthritis are not yet available for bimekizumab, but based on mechanism of action, we can assume it may work, and we anticipate future trials.
Molecular Signature Response Classifier
Dr. Curtis also reviewed data on the molecular signature response classifier (MSRC), a blood test that aims to predict non-responders to tumor necrosis factor inhibitors (TNFi) in patients with RA for whom methotrexate proved ineffective. The MSRC uses 19 gene expression biomarkers combined with four clinical features (i.e., anti-cyclic citrullinated peptide serostatus, sex, body mass index and patient global assessment) to generate a score on a continuous scale of 1 to 25. The higher the score, the greater the likelihood of nonresponse to TNFi therapy. The test is commercially available as PrismRA via direct online order through the manufacturer, with results available within two weeks. The manufacturer estimates the average out-of-pocket cost at approximately $75 and will assist if claims are denied. Insurance coverage for testing is evolving. Of note, lab-developed tests don’t require FDA clearance.
In a study evaluating RA biologic selection and treatment outcomes in a real-world cohort of patients with RA for whom methotrexate failed (n=212), the MSRC predicted TNFi non-response in 55% of patients and response in 45% of patients. When providers didn’t follow the recommendation of the blood test (i.e., the test recommended a non-TNFi but a TNFi was selected, likely due to insurance restrictions), the ACR50 response was only 10%. If the test recommendation was followed and a non-TNFi was selected, the ACR50 response was 35%, a statistically significant difference.6
“Reimbursement for this test is still in evolution,” said Dr. Curtis, “but this [test] is something you could choose to order for a patient when picking an RA drug. At this point, the test is most well studied in biologic-naive patients.”
Summary
New therapies and laboratory tests have the potential to profoundly affect patient care. Dr. Curtis highlighted the pluses and minuses, providing evidence-based guidance for the rheumatology community.
 Samantha C. Shapiro, MD, is an academic rheumatologist and an affiliate faculty member of the Dell Medical School at the University of Texas at Austin. She is also a member of the ACR Insurance Subcommittee.
Samantha C. Shapiro, MD, is an academic rheumatologist and an affiliate faculty member of the Dell Medical School at the University of Texas at Austin. She is also a member of the ACR Insurance Subcommittee.
References
- Morand EF, Furie R, Tanaka Y, et al. Trial of anifrolumab in active systemic lupus erythematosus. N Engl J Med. 2020 Jan 16;382(3):211–221.
- Rovin BH, Teng YKO, Ginzler EM, et al. Efficacy and safety of voclosporin versus placebo for lupus nephritis (AURORA 1): A double-blind, randomised, multicentre, placebo-controlled, phase 3 trial. Lancet. 2021 May 29;397(10289):2070–2080.
- Khanna D, Lin CJF, Furst DE, et al. Tocilizumab in systemic sclerosis: A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Respir Med. 2020 Oct;8(10):963–974.
- Roofeh D, Lin CJF, Goldin J, et al. Tocilizumab prevents progression of early systemic sclerosis-associated interstitial lung disease. Arthritis Rheumatol. 2021 Jul;73(7):1301–1310.
- Warren RB, Blauvelt A, Bagel J, et al. Bimekizumab versus adalimumab in plaque psoriasis. N Engl J Med. 2021 Jul 8;385(2):130–141.
- Strand V, Cohen SB, Curtis JR, et al. Clinical utility of therapy selection informed by predicted nonresponse to tumor necrosis factor-ɑ inhibitors: An analysis from the study to accelerate information of molecular signatures (AIMS) in rheumatoid arthritis. Expert Rev Mol Diagn. 2022 Jan;22(1):101–109.