ECD is a rare, potentially life-threatening condition, which recently has been better understood.2,6 Recent consensus guidelines have helped in both the diagnosis and management of this disease.2 Multiple therapeutic approaches have been used for ECD, but treatment is limited to Grade C and D evidence based on a lack of randomized controlled trials and few limited prospective therapeutic studies.2 Historically, such therapies as vinca alkaloids, anthracyclines, cyclophosphamide and high-dose chemotherapy with autologous stem cell transplantation, were utilized in small series with poor outcomes.2 Corticosteroids were found to be ineffective as monotherapy or in inducing remission; however, clinical experience supports their use to decrease acute inflammation.2
(click for larger image)
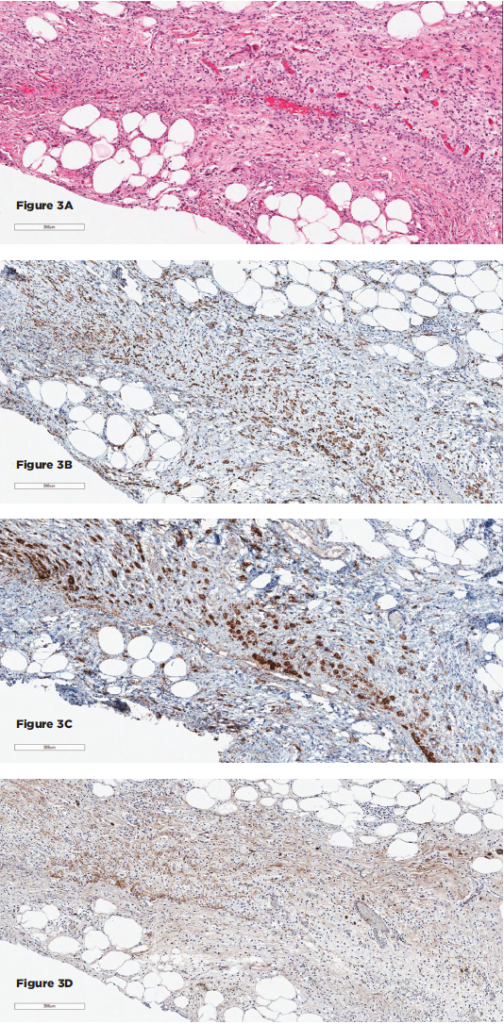
Figures 3A–D show the histopathologic findings for Patient 2. 3A–D show sections of the retroperitoneal lesion showing a fibrohistocytic lesion with foamy histiocytes on H&E (3A), positive for CD68 (3B) and BRAF (3C), negative for S100 and CD1a (not shown), and with only rare IgG4+ plasma cells (3D).
First-line therapy remains interferon therapy (IFN-α), based on multiple case series showing a survival benefit.2 However, interferon treatment has major limitations, which include the lack of efficacy, particularly in the presence of cardiovascular and CNS involvement, as well as an unfavorable side effect profile.7,8 Second-line therapies have included cladribine, sirolimus, infliximab, anakinra, tyrosine kinase inhibitors and vemurafenib.
Sirolimus (SRL) is an inhibitor of the mammalian target of rapamycin (mTOR) and has been used in treating malignancies and transplant rejection.3 An observation that a small number of ECD patients who have activating PIK3CA mutations resulting in mTOR pathway activation has led to sirolimus as a consideration in treatment of ECD.2,3,9
A recent open-label prospective trial by Gianfreda et al using sirolimus and prednisone was additionally based on the rationale of interference with the immune dysregulation seen in ECD.3 The study enrolled 10 patients. Eight had objective response or disease stabilization, and two had disease progression.3 The reported overall mortality was 20%.3
The number of cases reported using anti-tumor necrosis factor (TNF) therapies is limited. Dagna et al from Italy describe a 56-year-old man with a two-year history of recurrent pericardial effusion and acute cardiac tamponade who was successfully treated with infliximab 5 mg/kg every six weeks.10 Methotrexate was added to reduce the risk of possible autoantibodies.10
A second patient, a 60-year-old man with cardiac involvement, failed IFN-α because of side effects but had a good response to 5 mg/kg infliximab every six weeks.10