Since the mid-1980s, antiphospholipid (aPL) antibodies and their associated clinical manifestations have attracted great interest among clinicians and investigators. Indeed, the attention directed to aPL often exceeds that for other autoantibodies within the field of autoimmunity. Even in systemic lupus erythematosus (SLE), which is characterized by a multitude of specificities, the interest in this serological system remains high. The lupus anticoagulant (LAC) was first recognized more than 60 years ago, and its association with thrombosis and fetal loss was described in the mid-1970s.1-3 A dramatic boost in clinical interest came with the introduction of the anticardiolipin (aCL) test and subsequent efforts to link this test, as well as the LAC test, to a “new” condition, namely, antiphospholipid syndrome (APS).
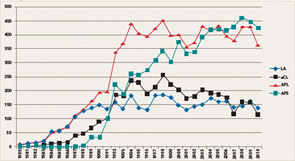
If the number of publications that use the keywords lupus anticoagulant, anticardiolipin antibodies, antiphospholipid antibodies, and antiphospholipid syndrome represents a measure of scientific interest, it is evident that an exponential increase in publications on these subjects began between 1984 and 1988 (see Figure 1). Indeed, investigators involved in the early phases of this work in the 1980s might be amazed today because, nearly three decades later, aPL antibodies and APS continue to attract a robust literature. Even if the number of publications may be showing a plateau, the high annual scientific output is impressive.
There are several reasons for the sustained interest in APS:
- The field has attracted clinicians and investigators from many disciplines, including rheumatology, hematology, obstetrics and gynecology, neurology, dermatology, and immunology.
- APS can have devastating and long-term debilitating consequences and usually affects otherwise healthy young individuals, particularly women.
- Beginning in the early 1990s, the development of in vivo mouse models, supported by in vitro studies, provided compelling evidence for a role of these antibodies in thrombosis, pregnancy loss, and thrombocytopenia.4-6
- APS is a multisystem disease, and several new pathogenic mechanisms have recently emerged to ignite interest in basic and translational research.
- Subclassification of the disorder and identifying its “true” clinical features based on solid evidence remains difficult.
- The optimal management of aPL antibody–related clinical manifestations, in particular, thrombosis and recurrent pregnancy loss, remains controversial.
- Importantly, testing for the various antibodies associated with APS has been the source of much controversy, particularly regarding the performance of different tests, measurement of antibody levels, and the extent of association of any serological finding with the various disease manifestations.
This article will summarize current data on traditional or “criteria” aPL antibody testing and discuss emerging assay methodologies and technologies. Our aim is to provide meaningful and accessible information so that practitioners understand the meaning of serological and functional tests, and to guide rheumatologists and other clinicians in their thinking as they face the challenge of diagnosing and managing patients suspected of having APS.
Criteria aPL Tests
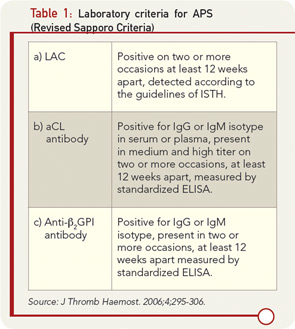
Current classification criteria for definite APS require the use of three “standardized” laboratory assays to detect aPL. These assays include aCL, both immunoglobulin (Ig) G and IgM; anti-β2glycoprotein I (anti-β2GPI) antibodies IgG and IgM; and/or LAC.7 These tests, when positive, represent criteria for diagnosis when at least one of the two major clinical manifestations (thrombosis or pregnancy losses) is present, according to the revised Sapporo criteria (see Table 1). Laboratory testing for aPL antibodies is one of the most problematic areas in the field of APS. The confirmation of diagnosis of the APS relies on laboratory tests because clinical manifestations such as thrombosis and pregnancy losses may occur for many reasons not related to the presence of aPL antibodies. Most important, patients with APS who have experienced thrombosis and/or pregnancy loss need a specific therapy that is often lifelong and must be personalized, requiring careful monitoring of additional risk factors to prevent recurrences of APS manifestations. Given the potential serious side effects of anticoagulant therapy, a solid diagnosis is essential in planning management.
Although international consensus guidelines for the determination of LAC have been published and revised, “standardized” tests for detection of aCL and anti-β2GPI have remained elusive. Furthermore, despite more than 7,000 publications related to the clinical use of aPL antibody tests, a consensus on clinical recommendations has been difficult to achieve. This difficulty appears related to suboptimal design in clinical studies and lack of laboratory standardization in areas such as: 1) units of measurement; 2) calibration curves; 3) determination of cut-off values; and 4) laboratories not performing the tests according to established guidelines. Significant interassay and interlaboratory variation in the results of both aCL and anti-β2GPI testing still exists, affecting the consistency of the diagnosis of APS.8
Over the years, international workshops have labored to standardize the laboratory test in this area. These workshops include the APL European Forum, the Australasian Anticardiolipin Working Party, the College of American Pathologists, the National External Quality Assessment Scheme, and the Standardization Subcommittee on Lupus Anticoagulant and Phospholipid-Dependent Antibodies of the International Society of Thrombosis and Hemostasis (ISTH). Although some laboratories can obtain reliable testing results, there is still wide interlaboratory variation despite efforts at standardization. This situation may result from laboratories performing aPL antibody assays with their own protocols or using commercial kits that do not conform to the proposed guidelines for these tests. Standardization of tests or reevaluation of standardization is important because APS is related to serious complications such as thrombosis and pregnancy loss; missing a diagnosis because of laboratory variability could have serious medical consequences. The use of semi- or fully automated analyzers and commercial kits instead of in-house assays poses additional challenges to the process of standardization.8
Criteria aPL Task Force and Work Recommendations
To address the challenges on aPL antibody testing described above, an international criteria aPL task force of researchers and scientific leaders in the field was formed prior to the 13th International Congress on Antiphospholipid Antibodies in Galveston, Texas, in April 2010 (APLA 2010). The task force was further divided into three subgroups that were charged by the congress chair to address, in an evidence-based manner, various topics related to the testing of aCL, anti-β2GPI, and LAC.
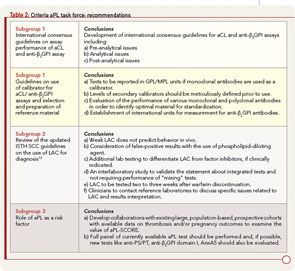
To accomplish its mission, the task force considered published information, the results of a survey distributed among congress attendees, and discussions that occurred during a special preconference workshop. On the basis of this approach, the task force reached several conclusions and proposed recommendations discussed here and summarized in Table 2; this information was recently published.9
Subgroup 1
Emerging new detection technologies and automated platforms with constantly evolving laboratory environments have made it necessary to update the standardization process and to revise existing protocols. Moreover, at present, there are no international consensus guidelines for aCL and anti-β2GPI. The task force members prepared a manuscript that included international guidelines centering on the characteristics of immunoassays for the measurement of aCL and anti-β2GPI antibodies.10 These guidelines are for the use of both consumers and manufacturers. The topics covered by guidelines include preanalytical issues (type of specimen), analytical issues (type and source of the antigen, method of calibration and quantitation, precision of the measurements, the issue of single or duplicate testing, interference testing, cut-off calculation, and expression of the result), and postanalytical issues (method of reporting results and interpretative comments).
Among other issues relevant to antibody testing, the use of monoclonal or polyclonal antibodies for assay calibration has been a source of controversy. A wet workshop organized at APLA 2010 compared the performance characteristics of currently available monoclonal antibodies: HCAL (IgG) and EY2C9 (IgM). This study identified large variability in results obtained with the use of these monoclonal antibodies, currently distributed by the Centers for Disease Control and Prevention.11 Also, there are no guidelines available concerning the preparation and cross-validation of secondary calibrators or the evaluation of available preparations. On careful review of the data, the task force recommended that levels of secondary calibrators should be carefully defined following accepted procedures prior to use. The task force also decided that any aCL tests using monoclonal preparation as a calibrator should report aCL titers in GPL/MPL units, a method currently used by the majority of laboratories worldwide.
The task force recommended an organized effort to evaluate further the performance of monoclonal and polyclonal antibodies and to identify optimal candidates for standardization. Once the candidate source is identified, the standard should be established according to current World Health Organization (WHO) guidelines to ensure its acceptance by regulatory authorities. Efforts focused on the development of international reference standards for IgG and IgM aCL and anti-β2GPI antibodies should be increased. In addition, a top priority of the task force will be to encourage all manufacturers to use the same calibrant material, whether human-derived polyclonal or monoclonal, so that this material can be designated as a WHO standard. This process could be facilitated by an internationally recognized organization. For the anti-β2GPI assay, there is no universal unit currently available. Therefore, the task force recommended the establishment of international units for measurement of anti-β2GPI antibodies. A work group has been assembled to carry out that objective, and a significant amount of work has already been completed.
Subgroup 2
Updated ISTH SCC guidelines on the use of LAC as a diagnosis of aPL antibodies incorporate a number of important issues. These issues include the following: definition of a “weak” LAC, as it is thought to be a confounding factor for diagnosis; false-positive screening assays secondary to the use of phospholipid-dilute reagents; embracing thrombin clotting time (TCT) and prothrombin time (PT) for the detection of LAC to determine the presence of heparin or warfarin interfering with test results; integrated testing systems; interpretative comments, as some factor inhibitors cause false-positive test results; and contacting reference laboratories for tests results and specific questions.12,13 Based on these considerations, the task force made recommendations described in Table 2 that were recently published.9
Subgroup 3
The group evaluated the possibility of using results of aPL antibody tests to calculate “risk factors” for APS-related clinical manifestations. Otomo and colleagues have described an antiphospholipid score (aPL-S), a weighted summation of results from LAC, aCL, anti-β2GPI, and anti–phosphatidylserine/prothrombin (anti–PS/PT) assays based on odds ratios. A higher prevalence of APS clinical manifestations was observed in patients with higher aPL-S, suggesting the use of a cluster analysis in the future to predict an APS risk factor. Also, when considering groups of tests, the risk of APS was found to be calculated as higher if the data were analyzed by cluster analysis or by combining the results of more than one test (aCL, anti-β2GPI, LAC).
Based on the limited data available to establish the use of aPL antibody tests as risk factors, the task force supported the idea of deriving a risk factor score but recommended expanding or confirming these preliminary observations by identifying and developing collaborations and by conducting studies with existing large, population-based, prospective cohorts with available data on thrombosis and/or pregnancy outcomes. In addition, the task force recommended that a full panel of current aPL antibody tests be included in any one of those studies (i.e., aCL and anti-β2GPI immunoassays and LAC assays). Furthermore, new tests such as antiprothrombin, anti–PS/PT, anti-β2GPI domain I, and annexin A5 (AnxA5) resistance (discussed below) should be studied as well.9
Noncriteria APL Tests: Hope for a Better Future on APL Testing?
As indicated above, the revised classification criteria for the diagnosis of APS includes positivity of at least one of the three criteria aPL antibody tests.7 However, the use of these tests may not guarantee full sensitivity and specificity to confirm an APS diagnosis. In clinical practice, there are indeed many “false positives” with aPL antibody tests, especially with the aCL ELISA, which can give positive results in clinical conditions aside from APS; these conditions include infectious diseases (e.g., syphilis), malignancies, and other autoimmune diseases. However, there are patients with clinical patterns strongly suggestive of APS but who are persistently negative for criteria tests. Additionally, the criteria aPL antibody tests may not identify “pathogenic” subpopulations of aPL antibodies.
Several autoantibodies have been demonstrated to bind directly to negatively charged phospholipids other than cardiolipin (individually or as a phospholipid mixture) or to other proteins in the coagulation cascade (i.e., prothrombin and/or phosphatidylserine-prothrombin complexes); antibodies can also interfere with anticoagulant activity of the AnxA5. However, the clinical and diagnostic utility of these newly developed assays as well as their standardization requires much further study. In some cases, these new assays lack standardization, and there are not international units of measurements.
A noncriteria aPL task force assembled prior to the APLA 2010 Congress was charged by the congress chair to address, in an evidence-based manner, the status of various new tests under development for confirmation of the diagnosis of APS. The results and recommendations of that task force are discussed here and were also recently published elsewhere.14
Noncriteria aPL Task Force Work and Recommendations
In addition to aCL, aPL antibodies are directed against negatively charged phospholipids. These antibodies include antiphosphatidic acid (anti-PA), antiphosphatidyinositol (anti-PI), antiphosphatidylserine (anti-PS), and antiphosphatidylglycerol. Furthermore, some antibodies can be detected by binding to a mixture of negatively charged phospholipids (APhL). Very few studies have been performed to determine the relevance of these antibody markers in clinical settings. Anti-PS and APhL have been the most extensively investigated in the settings of thrombosis and pregnancy morbidity, and both tests have been shown to be more specific for APS when compared with aCL.15-18
With regard to obstetrical manifestations of APS, various investigators have screened the sera of patients with recurrent pregnancy loss (RPL) to identify antibody specificities that might be missed if only aCL is tested. In a large retrospective study, Yetman and Kutteh determined the prevalence of aPL antibodies among 866 women with RPL compared with 288 controls. In this study, 150 of the women with RPL were positive for aCL (IgG and/or IgM), whereas 87 were negative for aCL, but positive for one of the other aPL antibodies.19 Hence, a significant number of the women with RPL would not have been identified if they had been tested only for aCL. In another study, the same group found anti-PS positivity in 49 of 872 women with RPL who were negative for aCL and LAC.20 Basic laboratory studies support the involvement of aPL other than aCL in obstetric APS: anti-PS antibodies have been shown to inhibit trophoblast development and invasion using an in vitro model system.21 These antibodies also may retard syncytiotrophoblast formation and decrease the synthesis of β-hCG.
Even for assays using the same reagents, however, the results of tests can be discordant, as there are no formal calibrators or accepted methods of detection. Hence, the current level of evidence does not warrant any changes to the current criteria and testing for anti-PA, anti-PI, and anti-PS antibodies in the initial diagnostic work-up for APS doesn’t appear clinically useful. Likely, anti-PS is the best candidate for further investigation, provided that an accepted and standardized method is in place.
Antibodies directed toward phosphatidylethanolamine (anti-PE) appear to occur particularly frequently in women with unexplained early fetal loss (UFL). Two studies have shown that the presence of anti-PE antibodies is a higher independent risk factor for early UFL than either aCL or anti-β2GPI antibodies.22,23 Moreover, anti-PE antibodies have been reported as the only aPL antibodies found in cases of UFL (73%). Regarding thrombosis, which is the other main clinical feature of APS, a multicenter study conducted within the framework of the European Forum on aPL antibodies found the prevalence of anti-PE was 15% in patients with unexplained venous thrombosis; this specificity was found mainly as the sole aPL antibody.24 At present, there is no accepted standardized method for the measurement of anti-PE, and the heterogeneity of these antibodies increases the difficulties in attaining such a goal. This problem significantly limits the utility of this assay. Hence, following current evidence, the evaluation of anti-PE antibodies can be suggested in patients with “seronegative” APS despite the absence of an accepted method for antibody detection. Further studies are necessary to establish the place of these antibodies in the diagnostic algorithm for APS, including standardization and proper validation of an anti-PE ELISA test and a prospective clinical study on a broad population with APS.
Anti-β2GPI antibodies are a heterogeneous population of antibodies with reactivity toward different epitopes on β2GPI.25 Among specificities to these epitopes, antibodies to domain I (DI) and anti-DI antibodies are more frequently associated with thrombosis and pregnancy morbidity compared with other anti-β2GPI antibodies; this finding has been confirmed in a recent double-blind multicenter study including 422 patients all positive for anti-β2GPI antibodies.26 However, since the role of anti-DI antibodies in pathogenesis has been demonstrated only in animal models, further studies are needed before the anti-DI assay can be added to the diagnostic guidelines.27
The current criteria aPL antibodies do not consider IgA isotypes for the aCL and anti-β2GPI tests. Several groups have investigated the prevalence of IgA aCL antibodies in SLE and APS patients and the relationship of these antibodies to clinical manifestations of APS. In unselected patients with SLE, the prevalence of increased titers of IgA aCL has been reported to vary from 1% to 44%.28,29 Interestingly, the ethnic background of patients may influence the distribution of aCL isotypes. Molina and colleagues compared the prevalence of IgA aCL in African-American, Afro-Caribbean, and Hispanic patients with SLE and found a higher rate in the Afro-Caribbean population (21% compared with 16% of the American-Americans and 14% of the Hispanic population). Furthermore, aCL IgA was the only isotype present in 82% of aCL-positive Afro-Caribbean patients.30
In another study, Cucurull and colleagues demonstrated the presence of IgA aCL in more than half of the patients with APS, although most of them were also positive for IgG and IgM; these findings suggest that testing for IgA would add little diagnostic information beyond that provided by IgG and IgM determinations.31 In the same study, the authors demonstrated an association between elevated levels of IgA aCL antibodies and clinical manifestations of APS. Interestingly, IgA aCL antibodies have been shown to be thrombogenic in mice.4 Thus, most of data indicate that isolated IgA aCL positivity is rare but may be associated with clinical manifestations of APS. Considering the low prevalence of IgA aCL positivity alone, IgA aCL testing should be performed only in cases where IgG and IgM aCL tests are negative but the suspicion of APS is high.
Several studies have investigated the association between IgA anti-β2GPI and clinical features of APS. Yamada and colleagues have found IgA anti-β2GPI positivity in a subgroup of women with recurrent pregnancy loss who were nevertheless negative for IgG anti-β2GPI.32 These antibodies likely bind to domain IV and domain V of β2GPI, which may contribute to thrombosis.33 Kumar and colleagues reported five cases of patients with clinical manifestations of APS but positivity only for IgA anti-β2GPI.34 Recently, a group of investigators from Dr. Pierangeli’s laboratory analyzed the prevalence of isolated IgA anti-β2GPI in three different cohorts: two SLE cohorts composed of 588 and 200 participants, respectively, and 5,098 individuals referred between January 2008 and March 2010 to the investigator’s laboratory for APS work-up (APLS). The authors found that 35, 15, and 25 participants from the three cohorts, respectively, were positive exclusively for IgA anti-β2GPI while negative for all the other aPL antibodies, including IgA aCL. Importantly, the majority of those patients (70% to 100%) had clinical features of APS. Furthermore, IgA anti-β2GPI antibodies have recently been found to be an independent risk factor for acute myocardial infarction and acute cerebral ischemia in populations without APS.35
In view of these findings, the task force recommended that IgA anti-β2GPI should be tested in the presence of clinical features of APS or SLE, particularly when other tests are negative. In addition, well-designed studies, which should include evaluation and comparison of multiple available assays in large populations of patients, are needed to confirm the diagnostic value of this test.
To address the challenges on aPL antibidy testing, an international criteria APL task force of researchers and scientific leaders in the field was formed prior to the International Congress on Antiphospholipid Antibodies.
Recent studies have investigated the clinical relevance of antibodies binding to human prothrombin alone (aPT-A) or to prothrombin-phosphatidylserine complex (anti–PS-PT). Funke and colleagues reported that aPS-PT conferred an odds ratio of 2.8:1 for venous thrombosis and 4.1:1 for arterial thrombosis in patients with SLE.36 Atsumi and colleagues showed that aPS-PT conferred an odds ratio for APS of 3.6:1 in 265 Japanese patients with systemic autoimmune diseases.37 In recent years, at least two prospective studies have shown that the presence of aPT-A is a predictor of recurrent thrombosis in APS patients.38,39 A 15-year longitudinal study has identified IgG aPT-A as the most useful predictor of thrombosis in SLE patients.40 These tests have been shown to have higher specificity but decreased sensitivity for the diagnosis of APS compared with aCL. Nevertheless, the task force recognized that the main problem with aPT-A and/or anti–PS-PT ELISAs is the lack of standardization despite efforts and international initiatives. Although these tests may be useful in screening for the risk for thrombosis, the task force did not recommend their inclusion as one of the laboratory criteria of APS at this time.
AnxA5 is a protein that can form a shield over phospholipids to block their availability for critical coagulation enzyme reactions. Previous studies have demonstrated that aPL antibodies can disrupt this anticoagulant shield and unmask anionic phospholipids, leading to thrombosis and pregnancy loss.40,41 The laboratory at Montefiore Medical Center in the Bronx, New York has developed a novel functional assay that measures the aPL antibody–mediated disruption of the AnxA5 shield. In particular, the assay measures the effect of patient plasma on the anticoagulant activity of AnxA5, adding plasma to a phospholipid suspension and then measuring the prolongation of coagulation time by AnxA5.42 The utility of this assay appears primarily in defining a subgroup of patients with clinical manifestations of APS in whom this disease mechanism occurs. The task force recognized that this assay is the first mechanistic assay developed to measure aPL antibodies but felt that additional data are needed before recommending AnxA5 resistance test as a standard component of aPL antibody testing panels.
Conclusions
The field of aPL antibody testing has evolved tremendously since the first aCL test was described by Harris and colleagues in 1983.43 Controversies and uncertainties still exist despite the significant efforts to standardize the aPL antibody tests. However, the scientific community is aware of these difficulties, and important initiatives are underway to hopefully enhance the performance of tests that are critical for the diagnosis of APS. Even with major laboratory research, the most important question a clinician faces today has remained almost the same since the discovery of aCL: What test(s) should I order to diagnose APS in a patient with thrombosis and/or pregnancy loss?
In the diagnosis and treatment of patients with APS, we believe strongly that clinicians should understand the scientific evidence supporting the use of a particular test and that laboratories should provide interpretative information and consultation to the physicians ordering the test. Such an approach would represent an important advance and hopefully improve the care of patients with these complicated, confusing, and often debilitating conditions.
Drs. Barilaro, Basra, Murthy, Willis, and Pierangel are with the Antiphospholipid Standardization Laboratory, division of rheumatology, department of internal medicine, University of Texas Medical Branch, Galveston, Texas. Dr. Willis is also with the department of microbiology, University of the West Indies, Kingston, Jamaica. Dr. Harris is with the Office of the Vice Chancellor, University of the West Indies. Disclosures: Drs. Pierangeli and Harris are co-owners of Louisville APL Diagnostics, Inc., a company that produces and distributes aPL antibody assays.
References
- Conley CL, Hartmann RC. A hemorrhagic disorder caused by circulating anticoagulant in patients with disseminated lupus erythematosus. J Clin Invest. 1952;31:621-622.
- Bowie EJ, Thompson JH Jr, Pascuzzi CA, Owen CA Jr. Thrombosis in systemic lupus erythematosus despite circulating anticoagulants. J Lab Clin Med. 1963;62:416-430.
- Nilsson IM, Asstedt B, Hedner U, et al. Intrauterine death and circulating anticoagulant “antithromboplastin.” Acta Med Scand. 1975;197:153-159.
- Pierangeli SS, Liu X, Barker JH, et al. Induction of thrombosis in a mouse model by IgG, IgM and IgA immunoglobulins from patients with the antiphospholipid syndrome. Thrombosis Haemost. 1995;74:1361-1367.
- Branch DW, Dudley DJ, Mitchell MD, et al. Immunoglobulin G fractions from patients with antiphospholipid antibodies cause fetal death in Balb/c mice: A model of autoimmune fetal loss. Am J Obstet Gynecol. 1990;163:210-216.
- Bakimer R, Fishman P, Blank M, et al. Induction of primary antiphospholipid syndrome in mice by immunization with a human monoclonal anticardiolipin antibody (H-3). J Clin Invest. 1992;89:1558-1563.
- Miyakis S, Lockshin MD, Atsumi T, et al. International consensus statement on an update of the classification criteria for definite antiphospholipid syndrome (APS). J Thromb Haemost. 2006;4;295-306.
- Pierangeli SS, Harris EN. A quarter of a century in anticardiolipin antibody testing and attempted standardization has led us to here, which is? Semin Thromb Hemost. 2008;334:313-328.
- Pierangeli S, Groot P, Dlott J, et al. “Criteria” aPL tests: Report of a task force and preconference workshop at the 13th International Congress on Antiphospholipid Antibodies, Galveston, TX. April 2010. Lupus. 2011;20:182-190.
- Lakos G, Favaloro, EJ, Harris EN, et al. International consensus guidelines on anticardiolipin and anti-β2glycoprotein I testing: A report from the APL task force at the 13th international congress on antiphospholipid antibodies. Arthritis Rheum. September 27, 2011. Epub ahead of print.
- Forastiero R, Papalardo E, Watkins M, et al. Evaluation of the performance of monoclonal and polyclonal antibody standards in different assays for the detection of antiphospholipid antibodies: Report of a wet workshop at the 13th International Congress on Antiphospholipid (aPL) antibodies. Arthritis Rheum. 2010; 62 (suppl):S946.
- Brandt JT, Triplett DA, Alving B, Scharrer I. Criteria for the diagnosis of lupus anticoagulants: an update. On behalf of the Subcommittee on Lupus Anticoagulant/Antiphospholipid Antibody of the Scientific and Standardisation Committee of the ISTH. Thromb Haemost. 1995;74:1185-1190.
- Pengo V, Tripodi A, Reber G, et al. Update of the guidelines for lupus anticoagulant detection. J Thromb Haemost. 2009;10:1737-1740.
- Bertolaccini ML, Amengual O, Atsumi T, et al. “Non-criteria” aPL tests: Report of a task force and preconference workshop at the 13th International Congress on Antiphospholipid Antibodies. Lupus. 2011;20:191-205.
- Rote NS, Dostal-Johnson D, Branch DW. Antiphospholipid antibodies and recurrent pregnancy loss: Correlation between the activated partial thromboplastin and antibodies against phosphatidylserine and cardiolipin. Am J Obstet Gynecol. 1990;163:575-584.
- Tebo AE, Jaskowski TD, Hill HR, Branch DW. Clinical relevance of multiple antibody specificità testing in anti-phospholpid syndrome and recurrent pregnancy loss. Clin Exp Immunol. 2008;154:332-338.
- Campbell AL, Pierangeli SS, Wellhausen S, Harris EN. Comparison of the effects of anticardiolipin antibodies from patients with the antiphospholipid syndrome and with syphilis on platelet activation and aggregation. Thromb Haemost. 1995;73:529-534.
- Harris EN, Pierangeli SS. A more specific ELISA assay for the detection of antiphospholipid. Clin Immunol Newsl. 1995;15: 26-28.
- Yetman DK, Kutteh WH. Antiphospholipid antibody panels and recurrent pregnancy loss: Prevalence of anticardiolipin antibodies compared with other antiphospholipid antibodies. Fertil Steril. 1996;66:540-546.
- Kutteh WH, Corey A, Jaslow CR. Antiphospholipid antibodies and recurrent pregnancy loss: Prevalence of anticardiolipin antibodies, the lupus anticoagulant, and antiphosphatidyl serine antibodies. Lupus. 2010;19:C156(abstract).
- Katsuragawa H, Kanzaki H, Inoue T, et al. Monoclonal antibody against phosphatidyl serine inhibits in vitro human trophoblastic hormone production and invasion. Biol Reprod. 1997;56:50-58.
- Gris JC, Quere I, Sanmarco M, et al. Antiphospholipid and antiprotein syndrome in non-thrombotic, non-autoimmune women with unexplained reccurrent primary early foetal loss. The Nimes Obstetricians and Haematologists Study – NOHA. Thromb Haemost. 2000;84:228-236.
- Sugi T, Matsubayashi H, Inomo A, et al. Antiphosphatidylethanolamine antibodies in recurrent early pregnancy loss and mid-to-late pregnancy loss. J Obstet Gynaecol Res. 2004;30:326-332.
- Sanmarco M, Gayet S, Alessi MC, et al. Antiphosphatidylethanolamine antibodies are associated with an increased odds ratio for thrombosis. A multicenter study with the participation of the European Forum on antiphospholipid antibodies. Thromb Haemost. 2007;97:949-954.
- de Laat B, Derksen RH, van Lummel M, et al. Pathogenic anti-beta2-glycoprotein I antibodies recognize domain I of beta2-glycoprotein I only after a conformational change. Blood. 2006;107:1916-1924.
- de Laat B, Pengo V, Pabinger I, et al. The association between circulating antibodies against domain I of beta2-glycoprotein I and thrombosis: An international multicenter study. J Thromb Haemost. 2009;7:1767-1773.
- Ioannou Y, Romay-Penabad Z, Pericleous C, et al. A novel concept for the in vivo inhibition of antiphospholipid antibody induced vascular thrombosis through the use of the antigenic target peptide domain I of b2glycoprotein I. J Thromb Haemost. 2009;7:833-842.
- Selva-O’Callaghan A, Ordi-Ros J, Monegal-Ferran F, et al. IgA anticardiolipin antibodies—relation with other antiphospholipid antibodies and clinical significance. Thromb Haemost. 1998;79:282-285.
- Weidmann CE, Wallace DJ, Peter JB, et al. Studies of IgG, IgM and IgA antiphospholipid antibodies isotope in systemic lupus erythematosus. J Rheumatol. 1988;15:74-79.
- Molina JF, Gutierrez-Urena S, Molina J, et al. Variability of anticardiolipin antibody isotype distribution in 3 geographic populations of patients with systemic lupus erythematosus. J Rheumatol. 1997;24:291-296.
- Cucurull E, Gharavi AE, Diri E, et al. IgA anticardiolipin and anti-beta2-glycoprotein I are the most prevalent isotope in African American patients with systemic lupus erythematosus. Am J Med Sci. 1999;318:55-60.
- Yamada H, Tsutsumi A, Ichikawa K, et al. IgA-class anti-beta2-glycoprotein I in women with unexplained recurrent spontaneous abortion. Arthritis Rheum. 1999;42:2727-2728.
- Martinez-Martinez LA, Aguilar-Valenzuela R, Seif A, et al. Do clinically relevant IgA anti-beta2-glycoprotein I (anti-b2-GPI) antibodies bind to DIV/V of b2GPI? Lupus. 2010;19:C130(abstract).
- Kumar S, Papalardo E, Sunkureddi P, et al. Isolated elevation of IgA anti-beta2glycoprotein I antibodies with manifestations of antiphospholipid syndrome: A case series of five patients. Lupus. 2009;18:1011-1014.
- Staub HL, Franck M, Ranzolin A, Norman GL. Beta2-glycoprotein I IgA antibodies and ischaemic stroke. Rheumatology (Oxford). 2006;6:104-106.
- Funke A, Bertolaccini ML. Atsumi T, et al. Autoantibodies to prothrombin-phosphatidylserine complex: Clinical significance in systemic lupus erythematosus (abstract). Arthritis Rheum. 1998;41:S240.
- Atsumi T, Icko M, Bertolaccini ML, et al. Association of autoantibodies against the phosphatidylserine-prothrombin complex with manifestations of the antiphospholipid syndrome and with the presence of lupus anticoagulant. Arthritis Rheum. 2000;43:1982-1993.
- Forastiero R, Martinuzzo M, Pombo G, et al. A prospective study of antibodies to beta2-glycoprotein I and prothrombin, and risk of thrombosis. J Thromb Haemost. 2005;3:1231-1238.
- Bizzarro N, Ghirardello A, Zampieri S, et al. Anti-prothrombin antibodies predict thrombosis in patients with systemic lupus erythematosus: A 15-year longitudinal study. J Thromb Haemost. 2007;5:1158-1164.
- Rand JH, WU XX, Quinn AS, et al. Human monoclonal antiphospholipid antibodies disrupt the annexin A5 anticoagulant crystal shield on phospholipid bilayers: Evidence from atomic force microscopi and functional assay. Am J Pathol. 2003;163:1193-1200.
- Hanly JG, Smith SA. Anti-bet2-glycoprotein I (GPI) autoantibodies, annexin V binding and the anti-phospholipid syndrome. Clin Exp Immunol. 2000;120: 537-543.
- Rand JH, Wuu XX, Lapinski R, et al. Detection of antibody mediated reduction of annexin A5 anticoagulant activity in plasmas of patients with the antiphospholipid syndrome. Blood. 2004;104:2783-2790.
- Harris EN. Anticardiolipin antibodies: Detection by radioimmunoassay and association with thrombosis. Lancet. 1983;2:1211-1214.