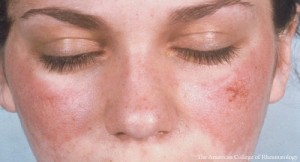
 BALTIMORE—In 1954, A. McGeHee Harvey, MD, professor and chair of the Department of Medicine, physician in chief, Johns Hopkins Hospital, Baltimore, and colleagues published a landmark paper in which they described 138 cases of systemic lupus erythematosus seen at that hospital and performed an extensive review of the literature. Almost exactly 70 years later at the same institution, the 19th Annual Johns Hopkins Advances in the Diagnosis and Treatment of Rheumatic Diseases Symposium featured a presentation titled, Important Clinical Outcomes in Systemic Lupus Erythematosus (SLE): How Are We Doing Now?
BALTIMORE—In 1954, A. McGeHee Harvey, MD, professor and chair of the Department of Medicine, physician in chief, Johns Hopkins Hospital, Baltimore, and colleagues published a landmark paper in which they described 138 cases of systemic lupus erythematosus seen at that hospital and performed an extensive review of the literature. Almost exactly 70 years later at the same institution, the 19th Annual Johns Hopkins Advances in the Diagnosis and Treatment of Rheumatic Diseases Symposium featured a presentation titled, Important Clinical Outcomes in Systemic Lupus Erythematosus (SLE): How Are We Doing Now?
Maintain a Low Disease State

Dr. Petri
Providing key insights into the topic, Michelle Petri, MD, MPH, MACR, professor of medicine and director of the Johns Hopkins Lupus Center, Johns Hopkins School of Medicine, Baltimore, began her presentation with a discussion of the Lupus Low Disease Activity State (LLDAS), which is defined by the following: 1) SLE Disease Activity Index 2000 (SLEDAI-2K) ≤4, with no activity in major organ systems, hemolytic anemia or gastrointestinal activity; 2) no new disease activity compared with prior assessment; 3) Safety of Estrogens in Lupus Erythematosus National Assessment (SELENA) SLEDAI physician global assessment ≤1; 4) steroid use of ≤7.5 mg daily of prednisone or equivalent; and 5) well-tolerated, standard maintenance doses of immunosuppressive drugs/approved biologics.1
Dr. Petri noted that LLDAS is an easier target to achieve than clinical remission in lupus and serves as an important outcome: Increased time spent in LLDAS is associated with a reduced rate of later organ damage, a lower risk of myocardial infarction and a lower risk of osteoporotic fracture.2
Concepts like LLDAS are significant because, despite progress made over the years, mortality rates in SLE remain high compared with the general population and SLE remains a leading cause of death for young women in the U.S.3 Outcomes in lupus remain poor compared with the expectations of clinicians and researchers for several reasons, according to Dr. Petri. One reason is that hydroxychloroquine, a medication with myriad positive effects in lupus, is not used optimally by providers or patients.
Hydroxychloroquine
In a study published this year by Nguyen et al., 660 patients from the Systemic Lupus Erythematosus International Collaborating Clinics (SLICC) Inception Cohort were evaluated with regard to hydroxychloroquine non-adherence (as measured by serum levels). The researchers found that 7% of patients demonstrated severe non-adherence (defined by a serum hydroxychloroquine level of <106 ng/mL or <53 ng/mL for doses of 400 or 200 mg/day, respectively) and that such non-adherence was independently associated with an increased risk of SLE flare, early damage and five-year mortality.4
According to Dr. Petri, ideal levels of hydroxychloroquine in whole blood should range from 750–1,000 mg/dL, and it is very reasonable for clinicians to monitor blood levels to ensure adherence and to confirm appropriate dosing.
Hydroxychloroquine, a medication with myriad positive effects in lupus, is not used optimally by providers or patients.
Overuse of Glucocorticoids
A second issue facing patients with lupus is that, as a whole, rheumatologists are still using too much prednisone for treatment, leading to poor outcomes for many patients. Evidence suggests that glucocorticoid use is an independent risk factor for damage accrual in SLE, and that development of irreversible organ damage associated with glucocorticoid treatment occurs in a dose-dependent fashion.5
For this reason, Dr. Petri explains, the “P” in prednisone stands for poison.
This helps explain why the EULAR recommendations for the management of SLE state that, in the chronic maintenance of lupus disease, glucocorticoid dose should be kept to less than 7.5 mg/day (prednisone equivalent) and, when possible, glucocorticoids should be withdrawn entirely.6
Morbidity & Mortality
Dr. Petri went on to explain that several other issues continue to impact morbidity and mortality in patients with lupus, including infections, thrombosis and cardiovascular disease. During the past several years, COVID-19 infection has been a particular concern in patients with lupus, both because of the potential for increased risk associated with the underlying disease and treatment and the impact that certain medications may have on dampening antibody production after COVID-19 vaccination.
Thrombosis is a particular problem in patients with antiphospholipid syndrome. In addition to anticoagulation with warfarin or a heparin product, Dr. Petri noted that evidence indicates potential benefits associated with hydroxychloroquine, aspirin, statins and vitamin D in these patients.
With regard to cardiovascular disease, it has been known for some time that lupus is an accelerant of atherosclerotic disease and that cardiac symptoms must be taken seriously in patients with lupus, irrespective of age. Dr. Petri and colleagues have developed a data-driven equation for cardiovascular risk in SLE, and this equation has been able to estimate the 10-year cardiovascular risk in patients with lupus better than traditional predictive models, such as the Framingham equation.7
Concluding Thoughts
Dr. Petri concluded her presentation by describing a few other roadblocks on the path to progress in treating lupus effectively. These include the threats to health posed by renal disease, malignancy and type 2 non-inflammatory symptoms.
Given new data that have emerged in recent years, Dr. Petri explained that a paradigm shift has occurred in which belimumab, the calcineurin inhibitor voclosporin and other treatments may be used in conjunction with mycophenolate mofetil or cyclophosphamide to prevent progression of lupus nephritis.
In terms of cancer risk in lupus, Zhang et al. performed meta analyses on 48 cohort studies and found an increased risk of cancer overall and of cancer-related death in patients with SLE compared with the general population. Moreover, SLE was a risk factor for 17 site-specific cancers, which include six digestive cancers, five hematologic cancers and cancer of the lung, larynx, cervix, vagina/vulva, kidneys, bladder, skin and thyroid.8
Regarding subtypes of lupus symptoms, Pisetsky et al. have helped define the concept of type 1 and type 2 symptoms of SLE. Type 1 symptoms include such features as rash, arthritis, alopecia and other manifestations that have a clear relationship to autoimmunity. Type 2 symptoms, such as fatigue, diffuse pain, cognitive dysfunction, sleep disturbance, anxiety/depression and brain fog, are non-inflammatory and don’t seem to respond well to immunosuppression.9 Recognition that both type 1 and 2 symptoms exist for many patients is essential to our ability to treat each patient as a whole person.
Although much work remains, Dr. Petri ended on a hopeful note. With advanced therapies, including CAR-T cell treatment, being explored in lupus each and every day, we can reasonably envision a future in which life for patients with lupus is longer, healthier and more fulfilling than what has been seen in years past.
Jason Liebowitz, MD, is an assistant professor of medicine in the Division of Rheumatology at Columbia University Vagelos College of Physicians and Surgeons, New York.
References
- Franklyn K, Lau CS, Navarra SV, et al. Definition and initial validation of a lupus low disease activity state (LLDAS). Ann Rheum Dis. 2016 Sep;75(9):1615–1621.
- Petri M, Magder LS. Comparison of remission and lupus low disease activity state in damage prevention in a United States systemic lupus erythematosus cohort. Arthritis Rheumatol. 2018 Nov;70(11):1790–1795.
- Singh RR, Yen EY. SLE mortality remains disproportionately high, despite improvements over the last decade. Lupus. 2018 Sep;27(10):1577–1581. Epub 2018 Jul 17.
- Nguyen Y, Blanchet B, Urowitz MB, et al. Association between severe non-adherence to hydroxychloroquine and SLE flares, damage, and mortality in 660 patients from the SLICC inception cohort. Arthritis Rheumatol. 2023 Jul 17.
- Apostolopoulos D, Kandane-Rathnayake R, Raghunath S, et al. Independent association of glucocorticoids with damage accrual in SLE. Lupus Sci Med. 2016 Nov 22;3(1):e000157.
- Fanouriakis A, Kostopoulou M, Alunno A, et al. 2019 update of the EULAR recommendations for the management of systemic lupus erythematosus. Ann Rheum Dis. 2019 Jun;78(6):736–745.
- Petri MA, Barr E, Magder LS. Development of a systemic lupus erythematosus cardiovascular risk equation. Lupus Sci Med. 2019 Oct 10;6(1):e000346.
- Zhang M, Wang Y, Wang Y, et al. Association between systemic lupus erythematosus and cancer morbidity and mortality: Findings from cohort studies. Front Oncol. 2022 May 4;12:860794.
- Pisetsky DS, Clowse MEB, Criscione-Schreiber LG, et al. A novel system to categorize the symptoms of systemic lupus erythematosus. Arthritis Care Res (Hoboken). 2019 Jun;71(6):735–741.


