Physicians are often puzzled by patients who suffer from a complex array of signs and symptoms that cannot easily be categorized into a single defined autoimmune or autoinflammatory disease. Such enigmatic conditions, although common in clinical practice, are rarely reported in the medical literature because they are so diverse and often present with nonspecific and vague findings. Not surprisingly, their pathogenesis remains a mystery. Moreover, possible therapies for these patients are difficult–if not impossible–to evaluate because most clinical trials are designed to follow patients with well-defined diseases and involve validated outcomes that often include specific markers.
The Case
As an example of such a complicated and perplexing situation, consider the following recently published case.1 The report described a 56-year-old woman diagnosed with chronic fatigue, fibromyalgia, weakness, headaches, difficulties concentrating, short-term memory impairment, and evidence of a demyelinating illness. Laboratory testing indicated the presence of high levels of antiadrenal, antistriated muscle, and antismooth muscle antibodies; increased rheumatoid factor titers; and elevated immunoglobin (Ig) G, IgM, and IgA immune complexes. The patient’s illness had begun 13 years earlier following the second dose of hepatitis B vaccine and was aggravated by the third vaccination. In addition, several years before vaccination, the patient had undergone uneventful silicone breast implantation. However, during the time between her second and third vaccination, she suffered a breast injury accompanied by evidence of local inflammation. Four years afterwards, she underwent removal of her breast implants. On histological examination, leaking of silicone and extensive calcification in both breasts was observed. In addition to removal of the implants, the patient was treated with intravenous IgG, and her condition gradually improved.
This illustrative case is notable for several clues that suggest a possible role of exposure to the hepatitis B vaccine and silicone leak that, together, produced an augmented adjuvant effect and led to the patient’s condition. In recent years, a large body of evidence has accumulated on the basis for vague conditions such as chronic fatigue and sick building syndromes. Subsequently, other poorly defined (and sometimes controversial) conditions—for example, siliconosis (relating to manifestations occurring following exposure to silicone) and postvaccination phenomena such as Gulf War syndrome (GWS) and the macrophagic myofasciitis syndromes (MMF)—have been suggested to have a common pathogenesis.2-5
Because these enigmatic diseases can display similarities in the presenting complex of signs and symptoms, a shared etiological common denominator is possible. In addition, accumulating data regarding immune adjuvant activity stimulated by various substances (e.g., infectious agents, silicone, aluminum salts) have been associated with the pathogenesis of each of these conditions. Thus, their comparable clinical presentation and plausible common mechanisms have led to the convergence of these conditions under a common syndrome called autoimmune (autoinflammatory) syndrome induced by adjuvants (ASIA).6
Autoimmunity Induced by Adjuvant
The worldwide prevalence of autoimmune and autoinflammatory diseases is determined by the interplay of genetic and environmental factors.7 The latter factors include infections, toxins, drugs, and others agents that can be linked by the occurrence of immune-mediated diseases as well as the nature of the clinical manifestations and their severity.8,9 The mechanisms by which these environmental factors trigger autoimmunity are diverse but, as a group, they may incorporate an adjuvant effect. An adjuvant is a substance that enhances the activation of the immune system, both the innate and the adoptive ones.10-12
For years, adjuvants have been used by physicians and scientists to boost a desirable immune response, either in experimental models or during medical interventions, most classically, immunization. The adjuvant effects encompass physical protection of the antigen from degradation, stimulation of innate immunity by toll-like receptors (TLRs) and non-TLRs sensors, antigen translocation to regional lymph nodes, and activation of the complement system.10 Consequently, adjuvants enable a longer exposure of the immune system to the antigen and prime the system for the production and activation of B and T cells, resulting in a more robust response.
Despite their ability to boost immune responses, in the past, adjuvants were generally considered to be inert materials that posed little or no independent threat to the host. Alas, animal studies as well as reports of human diseases have clearly demonstrated the ability of adjuvants to inflict diseases by themselves.10 One of the most studied adjuvants in this context is pristane. This adjuvant was found to be capable of inducing an autoimmune disease like systemic lupus erythematosus (SLE) in a murine model.13 Replicating features of human disease, pristane-induced lupus is characterized by the production of autoantibodies as well as organ damage (e.g., renal disease) that depends on the interferon (IFN)-I receptor signaling pathway. The adjuvant, squalene, can also induce arthritis in rats and the production of SLE- associated autoantibodies in mice.13,14
Aluminum is a widely used adjuvant that may produce immune activation and induce autoimmunity.10 This metal is encountered extensively in daily life through the soil, water, food, and pharmaceutical agents. Aluminum may be present in high amounts in dialysate, intravenous solutions, antacids, buffered aspirins, antidiarrheal products, bone cement, and alum-containing vaccines. The widespread use of aluminum was enhanced by the belief that it is nontoxic and rapidly excreted in the urine. Regrettably, it turns out that aluminum has several pathologic effects such as postdialysis encephalopathy, degenerative brain disorders, osteomalacia, cholestasis, ototoxicity, normo- or microcytic anemia, hemolytic anemia, disturbed erythropoiesis process, and inhibition of macrophage and leukocyte defensive mechanisms.15 The widely used adjuvant alum is hydrated potassium aluminum sulfate.
In a series of experiments, Shaw and Petrik studied the potential toxicity of aluminum hydroxide in male CD-1 mice.16 Aluminum-treated mice showed significant impairments in a number of motor functions as well as diminished spatial memory capacity. Histology demonstrated increased apoptosis of motor neurons and increases in reactive astrocytes and microglial proliferation within the spinal cord and cortex. Aluminum was observed in the cytoplasm of motor neurons with some neurons also testing positive for the presence of tau protein, a pathological hallmark of Alzheimer’s disease. Recent studies have indicated that alum can initiate cellular damage following the release of uric acid, a known trigger of the inflammasome and the activation of caspase 1 mediated downstream targets such as interleukin (IL)-1β, IL-18, and IL-33.12,17
Infectious agents are probably the environmental factors most closely associated with autoimmunity and autoimmune diseases.9 Recently, Rose proposed that, in addition to the known mechanisms by which infections elicit autoimmunity, an infectious adjuvant mechanism may play a role in immunopathogenesis.18 For instance, complete Freund’s adjuvant is a water-and-oil emulsion that includes killed mycobacterium. This adjuvant has been used to induce autoimmune diseases in many experimental models.18 Another example of the role of material for infectious organisms in eliciting autoimmunity is the requirement of the microbial component lipopoplysaccharide, used as an adjuvant, in certain disease models. The administration of lipopoplysaccharide with coxsackievirus B3 is used to overcome a genetic barrier and induce autoimmune myocarditis in a strain of mice genetically resistant to infection with coxsackievirus alone.18
Despite their ability to boost immune responses, in the past, adjuvants were generally considered to be inert materials that posed little or no independent threat to the host. Alas, animal studies as well as reports of human diseases have clearly demonstrated the ability of adjuvants to inflict diseases by themselves.
From these observations, it appears that the activation of the immune system by natural adjuvants (e.g., infectious agents) or pharmaceutical ones (e.g., vaccines containing alum or silicone), while usually followed by a desired activation of the immune system, could, in certain situations, trigger manifestations of autoimmunity or even autoimmune diseases itself.3,10 Yet the appearance of these diseases in association with exposure to an adjuvant is not as widespread as could be expected. This inconsistency may depend on the adjuvant itself, the genetic susceptibility of the exposed individual, and the rate of diagnosis and reporting such plausible associations.
Siliconosis: An Adjuvant Disease
Silicone-induced autoimmune phenomena was termed in the early 1990s, “the adjuvant disease.”2 At that time, various case reports of defined connective tissue diseases as well as a large cohort study, based on self-reported symptoms, suggested an association between silicone implants and defined connective tissue diseases.19 This association was later disputed by Janowsky et al in a meta-analysis performed that did not include the former study.20
Unlike the controversy regarding defined autoimmune diseases, a relationship between silicone implants and a constellation of signs and symptoms that do not fulfill a specific diagnosis was established several years later. In 2003, Vasey et al reported a statistically significant increase in body aches, joint pain, myalgia, fatigue, impaired cognition, and other symptoms following exposure to silicone.21 These findings were supported by another large study that reported an increase in similar symptoms among patients with silicone breast implants compared with a group of women who underwent reduction mammoplasties.2 The reported manifestations in both studies bear a resemblance to fibromyalgia and chronic fatigue syndrome. This was even further strengthened by the U.S. Food and Drug Administration’s finding that there is a correlation between fibromyalgia and ruptured silicone gel implants.2
Vaccines and Adjuvant Disease
Vaccines have been safely and effectively administered to humans and animals worldwide for 200 years, thereby enabling the elimination of many serious and life-threatening infectious diseases. Nevertheless, postvaccination adverse events, mostly transient and self-limited ones, have been reported. In addition, the occurrence of autoantibodies, inflammation, arthritis, neuronal damage, fatigue, cognitive impairment, and even overt autoimmune disease have been rarely observed among immunized people.3
A causal link between vaccines and autoimmunity was noted in 1976 during an outbreak of Guillain-Barré syndrome (GBS) that followed immunization with the “swine flu” vaccine.22 Causal relationships have also been accepted for transverse myelitis following an oral polio vaccine, autoimmune thrombocytopenia after measles-mumps-rubella, and arthritis following diphtheria-tetanus-pertussis.3
Additionally, a series of animal studies supported such a cause-and-effect interaction. Thus, immunization of young dogs resulted in the production of autoantibodies including lupus-associated ones. In diabetes-prone animals (e.g., NOD mice and BB rats) vaccination was associated with an increased incidence of diabetes and recently intraperitoneal immunization of salmon fish with oil-adjuvanted vaccines resulted in the production of autoantibodies thrombo-embolic disease, and immune-mediated glomerlulonephritis.3
The efficacy of most currently used vaccines depends on the presence of an adjuvant in conjunction with a foreign antigen corresponding to a component of an infectious agent.10,11 Adjuvants increase the protective and lasting immune response to the immunizing antigen and enable the decrease of the antigen amount and thereby the production of a larger amount of vaccines.10 One of the most evaluated post-vaccination autoimmune conditions is MMF, where a causal link to the vaccine adjuvant alum has been delineated.
Unraveling the adjuvant diseases pathogenesis may facilitate the search for preventive and therapeutic interventions.
MMF is a rare immune mediated muscles disease caused by deposition of aluminum, used to adjuvant different vaccines.5 MMF is characterized by a local active lesion at the site of inoculation as well as systemic signs and symptoms that resemble the ones described in relation to silicone exposure.5 These include myalgias, arthralgias, marked asthenia, muscle weakness, chronic fatigue, fever, and in some cases the appearance of a demyelinating disorder. Elevated creatine kinase and erythrocyte sedimentation rate as well as the appearance of autoantibodies, and myopathic electromyography changes also have been documented.5
The local lesion of MMF results from persistence of aluminum adjuvant at the site of inoculation for months and even 8–10 years following immunization.5 Intriguingly, the discrepancy between the wide application of aluminum hydroxide and the rarity of MMF recently has been resolved by the observations that MMF appears mainly in genetically susceptible subjects carrying the HLA–DRB1*01. This connection was first described in identical twin sisters diagnosed with MMF and later on in six of nine MMF patients, compared with 17% of 230 controls (O.R. of 9.8; 95% confidence interval 2.0–62.2).23
Another syndrome characterized by fatigue, neurological deficits, cognitive dysfunctions, and motor neuron disease is GWS. This syndrome may result from an adjuvant effect following multiple vaccinations performed over a short period of time. During the Gulf War, the veterans’ vaccination protocol included the anthrax vaccine, administered in a six-shot regimen and adjuvanted by aluminium hydroxide and squalene.10 In a relatively large study of 144 Gulf War-era veterans, 95% of overtly ill deployed GWS patients had antibodies to squalene. Furthermore, 100% of GWS patients immunized for service who did not deploy but had the same manifestations as those who did deploy had antibodies to squalene. In contrast, none of the control groups that incorporated patients with autoimmune diseases, healthy controls, and Persian Gulf veterans not showing signs of GWS had antibodies to squalene. Thus, although the pathogenesis of GWS is under scrutiny, the data assembled at this time highlight the possible role of adjuvants in this syndrome.
ASIA
Table 2: Suggested Criteria for the Diagnosis of ASIA
Major Criteria:
- Exposure to an external stimuli (infection, vaccine, silicone, adjuvant) prior to clinical manifestations
- The appearance of “typical” clinical manifestations:
- Myalgia, myositis, or muscle weakness
- Arthralgia and/or arthritis
- Chronic fatigue, nonrefreshing sleep, or sleep disturbances
- Neurological manifestations (especially associated with demyelination)
- Cognitive impairment, memory loss
- Pyrexia
- Dry mouth
- Removal of inciting agent induces improvement
- Typical biopsy of involved organs
Minor Criteria:
- The appearance of autoantibodies or antibodies directed at the suspected adjuvant
- Other clinical manifestations (i.e., irritable bowel syndrome)
- Specific HLA (e.g., HLA DRB1, HLA DQB1)
- Evolvement of an autoimmune disease (e.g., multiple sclerosis, Sjögren’s syndrome)
Source: J Autoimmun. 2011;36:4-8.
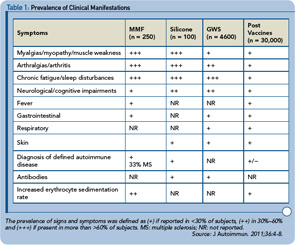
Taking it all together, it seems that enigmatic but nevertheless common and often disabling complaints can coincide in many individuals diagnosed with siliconosis, MMF, GWS, or postvaccination events (see Table 1). Additionally, in up to 35% of these patients, autoimmunity (e.g., autoantibodies) or an overt autoimmune disease may eventually be diagnosed. A noteworthy common denominator is that the exposure to a component that comprises an adjuvant effect can be documented in each of those medical conditions. These phenomena can occur weeks and even or years following exposure to a culprit agent. Moreover, genetic links observed in animal models, and in the human disease MMF, bring about the notion that the adjuvant effect promotes the appearance of an adjuvant disease in subjects who are genetically susceptible or in those who encounter an additional trigger, such as the effect of another deleterious environmental factor (e.g., infectious agent) or co-exposure to more than one adjuvant.
Last but not least, these medical conditions were recently encompassed as ASIA. Looking back at the numerous reports in the last four or even five decades, one might ask if this is actually a new syndrome. Apparently, physicians have observed these phenomena for years, but the lack of definition remained a major obstacle in diagnosing, treating, and conducting basic as well as clinical studies.
Perhaps the most novel aspect of this new syndrome is its spacious view of these comparable diseases as well as improvement of their definition and diagnosis utilizing major and minor criteria (see Table 2). This innovative characterization may provide physicians a better way to understand to the immune consequences of environmental adjuvants and advance the diagnosis of patients suffering from the adjuvant diseases. Moreover, unraveling the adjuvant diseases pathogenesis may facilitate the search for preventive and therapeutic interventions such as immune modulation of the adjuvant pathways (e.g., TLRs , inflammasome, and others).
Dr. Shoenfeld is head of the Zabludowicz Center for Autoimmune Diseases, Sheba Medical Center (affiliated with Tel-Aviv University), Tel-Hashomer, Israel. Dr. Levine is a senior physician in the Zabludowicz Center.
References
- Agmon-Levin N, Shoenfeld Y. Chronic fatigue syndrome with autoantibodies—the result of an augmented adjuvant effect of hepatitis-B vaccine and silicone implant. Autoimmun Rev. 2008;8:52-55.
- Hajdu SD, Agmon-Levin N, Shoenfeld Y. Silicone and autoimmunity. Eur J Clin Invest. 2011;41:203-211.
- Agmon-Levin N, Paz Z, Israeli E, Shoenfeld Y. Vaccines and autoimmunity. Nat Rev Rheumatol. 2009;5:648-652.
- Hotopf M, David A, Hull L, Ismail K, Unwin C, Wessely S. Role of vaccinations as risk factors for ill health in veterans of the Gulf War: Cross sectional study. BMJ. 2000;320:1363-1367.
- Israeli E, Agmon-Levin N, Blank M, Shoenfeld Y. Macrophagic myofaciitis a vaccine (alum) autoimmune-related disease. Clin Rev Allergy Immunol. 2010 Sep 30. [Epub ahead of print]
- Shoenfeld Y, Agmon-Levin N. ‘ASIA’- autoimmune/auto inflammatory syndrome induced by adjuvant. J Autoimmun. 2011;36(1):4-8.
- Shapira Y, Agmon-Levin N, Shoenfeld Y. Defining and analyzing geoepidemiology and human autoimmunity. J Autoimmun. 2010;34:J168-J177.
- Shoenfeld Y, Zandman-Goddard G, et al. The mosaic of autoimmunity: Hormonal and environmental factors involved in autoimmune diseases—2008. Isr Med Assoc J. 2008;10:8-12.
- Kivity S, Agmon-Levin N, Blank M, Shoenfeld Y. Infections and autoimmunity. Trends Immunol. 2009;30:409-414.
- Israeli E, Agmon-Levin N, Blank M, Shoenfeld Y. Adjuvants and autoimmunity. Lupus. 2009;18:1217-1225.
- Marrack P, McKee AS, Munks MW. Towards an understanding of the adjuvant action of aluminium. Nat Rev Immunol. 2009;9:287-293.
- Kool M, Soullié T, van Nimwegen M, et al. Alum adjuvant boosts adaptive immunity by inducing uric acid and activating inflammatory dendritic cells. J Exp Med. 2008;14;205:869-882.
- Reeves WH, Lee PY, Weinstein JS, Satoh M, Lu L. Induction of autoimmunity by pristane and other naturally occurring hydrocarbons. Trends Immunol. 2009;30:455-464.
- Santoro D, Stella M, Montalto G, Castelli S. Lupus nephritis after hepatitis B vaccination: An uncommon complication. Clin Nephrol. 2007;67:61-63.
- Lerner A. Aluminum is a potential environmental factor for Crohn’s disease induction: Extended hypothesis. Ann NY Acad Sci. 2007;1107:329-345.
- Shaw CA, Petrik MS. Aluminum hydroxide injections lead to motor deficits and motor neuron degeneration. J Inorg Biochem. 2009;103:1555-1562.
- Hornung V, Bauernfeind F, Halle A, et al. Silica crystals and aluminum salts activate the NALP3 inflammasome through phagosomal destabilization. Nat Immunol. 2008;9:847-856.
- Rose NR. Autoimmunity, infection and adjuvants. Lupus. 2010;19:354-358.
- Hennekens CH, Lee IM, Cook NR, et al. Self-reported breast implants and connective-tissue diseases in female health professionals. A retrospective cohort study. JAMA. 1996;275:616-621.
- Janowsky EC, Kupper LL, Hulka BS. Meta-analyses of the relation between silicone breast implants and the risk of connective-tissue diseases. N Engl J Med. 2000; 342:781-790.
- Vasey FB, Zarabadi SA, Seleznick M, Ricca L. Where there’s smoke there’s fire: The silicone breast implant controversy continues to flicker: A new disease that needs to be defined. J Rheumatol. 2003;30: 2092-2094.
- Lasky T, Terracciano GJ, Magder L, et al. The Guillain-Barre syndrome and the 1992-1993 and 1993-1994 influenza vaccines. N Engl J Med.1998;339:1797-1802.
- Guis S, Pellissier JF, Nicoli F, et al. HLA-DRB1*01 and macrophagic myofasciitis. Arthritis Rheum. 2002; 46:2535-2537.