 When Moll and Wright first described the spondyloarthritides in the early 1970s, the archetype of the group was ankylosing spondylitis (AS).1 The shared clinical features of the spondyloarthritides were sacroiliitis; asymmetric large joint peripheral arthritis; psoriasis or psoriaform skin lesions, including keratoderma blennorrhagica; uveitis; and bowel inflammation.
When Moll and Wright first described the spondyloarthritides in the early 1970s, the archetype of the group was ankylosing spondylitis (AS).1 The shared clinical features of the spondyloarthritides were sacroiliitis; asymmetric large joint peripheral arthritis; psoriasis or psoriaform skin lesions, including keratoderma blennorrhagica; uveitis; and bowel inflammation.
Moll and Wright described five clinical subgroups of psoriatic arthritis (PsA), with pure axial involvement in about 5% of cases. However, recent reports from Dafna Gladman’s group in Toronto have described co-existing peripheral and axial involvement (defined by symptoms and/or radiography) in over 50% of cases with increasing axial disease as time progresses.2,3 With the introduction of the term axial spondyloarthritis (axSpA) some confusion has arisen: This article discusses radiographic axSpA (AS) and axSpA in PsA, referred to as axPsA.
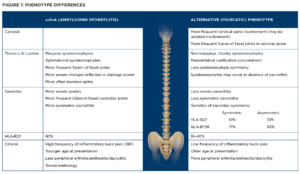
AxPsA can have two radiographic phenotypes: the classic axSpA (AS) phenotype and an alternative phenotype characterized by fewer symptoms, less symmetry of axial radiographic features, less involvement of the sacroiliac joints, relatively more involvement of the cervical spine and morphologically different syndesmophytes.4
Moreover, the clinical presentation of axPsA differs from that of axSpA: Patients tend to be older at presentation, with less back pain, less inflammatory back pain, less limitation of movement and less emphasis on male predominance.5
Genetics
Genetic differences also exist. Although human leukocyte antigen B27 (HLA-B27) is the predominant gene in inflammatory spinal disease, it is found less often in patients with axPsA. HLAB* 08 and HLA-B*38 are associated with axPsA, but not the ERAP1 and ERAP2 genes found in axSpA.6 These differences between the classic and alternative phenotypes are summarized in Figure 1.
The exact proportion of the classic and the alternative phenotypes in axPsA has yet to be determined, but because the phenotypes are likely driven by HLAB27 and because the proportion of patients with HLA-B27 in axPsA is between 19% and 40%, the likely ratio is in favor of the alternative phenotype.5,7,8
Implications for Clinical Practice
What is the importance of this for clinical practice? Does it really matter to clinicians? Yes, it does. Many of our patients with axPsA will go unrecognized, partly because peripheral musculoskeletal manifestations may predominate, but partly because the axial disease is less symptomatic. Even if the clinician routinely X-rays the sacroiliac joints, a certain proportion of axPsA will be missed because the disease may be visible only on magnetic resonance imaging and not plain radiographs and because spondylitis may occur in the absence of sacroiliitis in potentially up to a third of all patients with axial involvement in PsA.8
Classification Criteria
Further, if clinicians rely on established classification criteria for diagnosis—as is often the case in the absence of diagnostic criteria—the current spondyloarthritis criteria are not fit for the purpose from the point of view of axPsA. Consider the Assessment of Spondyloarthritis International Society (ASAS) criteria for axSpA.9
The stem for these criteria is three or more months of back pain starting before the age of 45. As indicated above, patients with axPsA tend to be older and less often report back pain. With regard to imaging, spondylitis may occur in the absence of sacroiliitis in axPsA, so patients may not fulfill this arm of the criteria. For the clinical arm, the presence of HLA-B27 is required, yet this is often not present in axPsA.
A study currently underway is re-examining the validity of the ASAS criteria (CLASSIC: Classification of Axial Spondyloarthritis Inception Cohort; NCT03993847), which may help, although the definition of axial involvement in PsA may be a problem.
In summary, axial disease cannot be ruled out in PsA without sacroiliac and spinal radiographs, irrespective of symptoms. As yet, we have no data regarding the difference in expression of axial involvement using MR, but it will be fascinating to see just such a comparative study using this alternative imaging, comparing non-radiographic and radiographic axSpA and axPsA. More information is needed on the alternative, psoriatic, phenotype—genetics, natural history, assessment, impact and response to treatment—but an urgent need is for validated classification criteria.
Treatment
The second major consequence of this heterogeneity in expression of axial disease concerns treatment. Most therapeutic clinical trials in patients with axSpA have not identified subtypes of axial disease. Currently, treatment recommendations for axPsA are borrowed from these axSpA studies, and include tumor necrosis factor (TNF) inhibitors and interleukin (IL) 17 inhibitors.10 Studies of IL-12/23 and IL-23 inhibitors have not shown benefit in axSpA.11,12
Only one study specifically targeted to axPsA showed a positive result for IL-17 inhibition, although the limitations of disease definition mentioned above provide some limitations on interpretation of this study.13 The heterogeneity of axial expression provides some uncertainty about the benefit of these monoclonal antibodies in axPsA.
Given the heterogeneity of expression, could it be possible that the alternative (psoriatic) phenotype will respond differently to treatment than the classic phenotype? Two sub-analyses of trials with, first, an IL-12/23 antibody and, second, with an IL-23 antibody provide a hint that the alternative phenotype might respond differently to these drugs.14,15
However, any conclusions on the efficacy of these antibodies in the alternative axPsA phenotype must be regarded with caution, for the following reasons. First, the studies were not primarily designed to look at the efficacy of these drugs in axPsA. Second, as noted above, in the absence of a validated definition of axPsA, it is difficult to know exactly what sort of patients were included as axPsA, although patients in the IL-23 studies were required to have radiographic sacroiliitis.
Third, and most importantly, the outcome measure used to define efficacy, the Bath Ankylosing Spondylitis Disease Activity Index (BASDAI), although standard for trials in axSpA, is not appropriate on its own for studies in patients who have a significant amount of peripheral arthritis, as was the case in these studies. Even selecting out the specific spine-focused questions of the BASDAI and examining response using only these items may be unreliable in patients with concomitant peripheral disease.16
Developing a New Definition of axPsA
Recognizing the clinical, radiographic and genetic differences of the psoriatic phenotype has prompted a joint effort by ASAS and the Group for Research and Assessment of Psoriasis and Psoriatic Arthritis (GRAPPA) to develop a new definition of axPsA, the AXIS trial (NCT04434885).17 This multinational, multicenter, cross-sectional study of 400 patients with PsA presenting with peripheral involvement will collect clinical, imaging and laboratory findings, with the aim of developing universally accepted classification criteria for axPsA.
This collaborative effort has already received expert consensus; in an online discrete choice experiment, physicians overwhelmingly opted for mandatory positive axial imaging as a classification criterion for axPsA.18 Hopefully this will satisfy the urgent need for an evidence-based and widely accepted definition of axial involvement in PsA that will allow us to define a homogeneous group of patients in which appropriate epidemiologic, clinical and interventional studies can be performed.
Similarly, the ongoing phase 4 STAR study (NCT04929210) will compare the efficacy of guselkumab, an IL-23 inhibitor, with placebo, in patients with peripheral arthritis who meet the Classification Criteria for Psoriatic Arthritis (CASPAR) and have inflammation of the spine and/or sacroiliac joint detected by centrally read magnetic resonance imaging. This study will help define axPsA and examine the efficacy of this drug in both the classic and alternative phenotypes of axial disease, thus helping resolve the uncertainties identified above.
 Philip Helliwell, DM, PhD, FRCP, is the professor of clinical rheumatology, Leeds Institute of Rheumatic and Musculoskeletal Medicine, University of Leeds, England.
Philip Helliwell, DM, PhD, FRCP, is the professor of clinical rheumatology, Leeds Institute of Rheumatic and Musculoskeletal Medicine, University of Leeds, England.
Disclosures
Dr. Helliwell has received consulting fees from Eli Lilly and fees for educational services from AbbVie, Amgen, Novartis, Pfizer and Janssen.
References
- Wright V, Moll JMH. Seronegative Polyarthritis. Amsterdam: North Holland Publishing Co. 1976.
- Chandran V, Barrett J, Schentag CT, et al. Axial psoriatic arthritis: Update on a longterm prospective study. J Rheumatol. 2009 Dec;36(12):2744–2750.
- Chandran V, Tolusso DC, Cook RJ, Gladman DD. Risk factors for axial inflammatory arthritis in patients with psoriatic arthritis. J Rheumatol. 2010 Apr;37(4):809–815.
- Helliwell PS, Hickling P, Wright V. Do the radiological changes of classic ankylosing spondylitis differ from the changes found in the spondylitis associated with inflammatory bowel disease, psoriasis, and reactive arthritis? Ann Rheum Dis. 1998 Mar;57(3):135–140.
- Feld J, Ye JY, Chandran V, et al. Is axial psoriatic arthritis distinct from ankylosing spondylitis with and without concomitant psoriasis? Rheumatology (Oxford). 2020 Jun 1;59(6):1340–1346.
- Eder L, Chandran V, Pellet F, et al. Human leucocyte antigen risk alleles for psoriatic arthritis among patients with psoriasis. Ann Rheum Dis. 2012 Jan;71(1):50–55.
- Coates LC, Baraliakos X, Blanco FJ, et al. The phenotype of axial spondyloarthritis: Is it dependent on HLA-B27 status? Arthritis Care Res (Hoboken). 2021 Jun;73(6):856-860.
- Jadon DR, Sengupta R, Nightingale A, et al. Axial disease in psoriatic arthritis study: Defining the clinical and radiographic phenotype of psoriatic spondyloarthritis. Ann Rheum Dis. 2017 Apr;76(4):701–707.
- Rudwaleit M, van der Heijde D, Landewé R, et al. The development of Assessment of SpondyloArthritis international Society classification criteria for axial spondyloarthritis (part II): Validation and final selection. Ann Rheum Dis. 2009 Jun;68(6):777–783.
- Coates LC, Soriano E, Corp N, et al. OP0229 The Group for Research and Assessment of Psoriasis and Psoriatic Arthritis (GRAPPA) treatment recommendations 2021. Ann Rheum Dis. 2021;80(Suppl 1):139–140.
- Deodhar A, Gensler LS, Sieper J, et al. Three multicenter, randomized, double-blind, placebo-controlled studies evaluating the efficacy and safety of ustekinumab in axial spondyloarthritis. Arthritis Rheumatol. 2019 Feb;71(2):258–270.
- Baeten D, Østergaard M, Wei JCC, et al. Risankizumab, an IL-23 inhibitor, for ankylosing spondylitis: Results of a randomised, double-blind, placebocontrolled, proof-of-concept, dose-finding phase 2 study. Ann Rheum Dis. 2018 Sep;77(9):1295–1302.
- Baraliakos X, Gossec L, Pournara E, et al. Secukinumab in patients with psoriatic arthritis and axial manifestations: Results from the double-blind, randomised, phase 3 MAXIMISE trial. Ann Rheum Dis. 2021 May;80(5):582–590.
- Mease PJ, Helliwell PS, Gladman DD, et al. Efficacy of guselkumab on axial involvement in patients with active psoriatic arthritis and sacroiliitis: A post-hoc analysis of the phase 3 DISCOVER-1 and DISCOVER-2 studies. Lancet Rheumatol. 2021 Oct 1;3(10):e715–e723.
- Helliwell PS, Gladman DD, Chakravarty SD, et al. Effects of ustekinumab on spondylitis-associated endpoints in TNFinaïve active psoriatic arthritis patients with physician-reported spondylitis: Pooled results from two phase 3, randomised, controlled trials. RMD Open. 2020 Feb;6(1) e001149.
- Reddy S, Husni M, Scher J, et al. Use of the BASDAI in psoriatic arthritis patients with and without axial disease. Meeting Abstract. ACR Convergence 2020. Abstract 0344. 2020 Nov 5–9.
- Poddubnyy D, Baraliakos X, Van den Bosch F, et al. Axial involvement in psoriatic arthritis cohort (AXIS): The protocol of a joint project of the Assessment of SpondyloArthritis International Society (ASAS) and theGroup for Research and Assessment of Psoriasis and Psoriatic Arthritis (GRAPPA). Ther Adv Musculoskelet Dis. 2021 Dec 18;13:1759720×211057975.
- Poddubnyy D, Mease PJ, Van den Bosch F, et al. AB0824 Which parameters are relevant in the identifying axial involvement in psoriatic arthritis?—Results of a survey among ASAS and GRAPPA members. Ann Rheum Dis. 2020;79(Suppl1):1715–1716.