The availability of new medications to treat axial spondyloarthritis (axSpA), including medications recently approved by the U.S. Food and Drug Administration to treat ankylosing spondylitis (AS)—secukinumab and ixekizumab—as well as new evidence on tapering and discontinuing biologics, magnetic resonance imaging (MRI) and radiograph imaging are the driving forces behind the release of a revised guideline for ankylosing spondylitis. The new guideline, “2019 Update of the American College of Rheumatology/Spondylitis Association of America/Spondyloarthritis Research and Treatment Network Recommendations for the Treatment of Ankylosing Spondylitis and Nonradiographic Axial Spondyloarthritis [nr-axSpA],” is now available online.1
The availability of new medications to treat axial spondyloarthritis (axSpA), including medications recently approved by the U.S. Food and Drug Administration to treat ankylosing spondylitis (AS)—secukinumab and ixekizumab—as well as new evidence on tapering and discontinuing biologics, magnetic resonance imaging (MRI) and radiograph imaging are the driving forces behind the release of a revised guideline for ankylosing spondylitis. The new guideline, “2019 Update of the American College of Rheumatology/Spondylitis Association of America/Spondyloarthritis Research and Treatment Network Recommendations for the Treatment of Ankylosing Spondylitis and Nonradiographic Axial Spondyloarthritis [nr-axSpA],” is now available online.1
AxSpA (including both AS and nr-axSpA) is the main form of chronic inflammatory arthritis affecting the axial skeleton. As many as 2.7 million adults may be affected by the disease, which is characterized by back and hip pain, peripheral joint pain and fatigue.2
The 2019 guideline includes 86 recommendations presented by the ACR, in partnership with the Spondylitis Association of America (SAA) and the Spondyloarthritis Research and Treatment Network (SPARTAN), to provide updated and new guidance for the management of patients with AS and nr-axSpA in the areas of pharmacologic and non-pharmacologic treatment options, AS-related co-morbidities, disease activity assessment, imaging and screening.
“New medications to treat axial spondyloarthritis, most notably interleukin 17A (IL-17A) inhibitors, secukinumab and ixekizumab, are now supported by large, published clinical trials that demonstrated efficacy in AS, so we thought it was important to update the recommendations to accommodate this new information,” says Michael Ward, MD, lead investigator of the guideline update.3,4
Early studies on tofacitinib and an expanding number of tumor necrosis factor inhibitor (TNFi) biosimilars, as well as recommendations on some other topics, such as imaging, were also considered in the update.
Guideline Updates at a Glance
To revise the guideline, Dr. Ward and the panel of co-authors updated the systematic literature review for 20 clinical questions on pharmacologic treatment addressed in the 2015 guideline and conducted a new literature review for 26 new questions on pharmacologic treatment, treat-to-target strategies and use of imaging. New questions addressed the use of secukinumab, ixekizumab, tofacitinib, TNFi biosimilars and biologic tapering and discontinuation, among other factors. Grading of Recommendations, Assessment, Development and Evaluation (GRADE) methodology was used to assess the quality of evidence and formulate recommendations. At least 70% agreement among the guideline voting panel was required for a recommendation to be included in the guideline.
The 2019 recommendations for AS and nr-axSpA are similar to the earlier guideline. Many of the recommendations are conditional instead of strong, driven mainly by the fact that little evidence in the literature supports the recommendation and future evidence may contradict it. Among the recommendations:
• TNFi’s are recommended over secukinumab or ixekizumab as the first biologic to be used;
• Secukinumab or ixekizumab is recommended over the use of a second TNFi in patients with a primary nonresponse to the first TNFi;
• Secukinumab and ixekizumab are favored over tofacitinib;
• Co-administration of low-dose methotrexate with a TNFi is not recommended, nor is a strict treat-to-target strategy, or discontinuation or tapering of biologics as a standard approach in patients with stable disease;
• Sulfasalazine is recommended only for persistent peripheral arthritis when TNFi’s are contraindicated; and
• For patients with unclear disease activity, spine or pelvis MRI could aid assessment. Routine monitoring of radiographic changes with serial spine radiographs is not recommended.
Please refer to the full guideline for a complete list of recommendations—and whether they are strongly recommended, conditionally recommended, conditionally recommended against or strongly recommended against—for each condition.
Practice Implications
Dr. Ward further explains three key guideline updates and how they will affect patient treatment.
Sequencing biologics for patients with active AS despite non-steroidal anti-inflammatory drug (NSAID) usage: For these patients, the recommendations are to first treat with a TNFi, provided there are no contraindications, Dr. Ward explains. “If there is a good response to the first TNFi, but effectiveness is lost over time, the recommendation is to treat with a different TNFi. If there is little or no response to the first TNFi, the recommendation is to treat with an IL-17 inhibitor. There is no preference for using any particular TNFi, but this is not the case if the patient has particular co-morbid conditions, such as recurrent uveitis or inflammatory bowel disease, when TNFi monoclonal antibodies should be used.”
Whether to taper or discontinue biologics in the setting of remission: For patients in sustained remission, the recommendation against tapering of biologics as a standard approach is conditional, Dr. Ward explains, noting that treatment is often needed long-term, and the evidence at present does not support that tapering after a certain amount of time in remission will not result in return of symptoms. He says that “as a standard approach” is an important qualifier, because tapering could be considered in some patients with shared decision making.
“This is why the recommendation is conditional,” he says. “The recommendation against discontinuation of biologics was also conditional, because the limited evidence suggests symptoms recur in many patients. Thus, many patients may want to stay on their medication to ensure this does not happen.”
When to obtain imaging, particularly when results would likely lead to treatment changes: Because symptoms and the physical exam can be nonspecific, Dr. Ward says it is occasionally difficult to know if new or ongoing pain and stiffness in the spine or pelvis represents ongoing disease activity in patients who are already taking a biologic. In these cases, MRI was conditionally recommended because finding evidence of inflammation on imaging in these cases may support a change in medication or in dose.
Alternatively, the recommendation is against obtaining MRIs to look for occult inflammation in patients who are clinically doing well. The guideline also includes a conditional recommendation against routine X-rays of the spine or pelvis on a regularly scheduled basis to monitor progression, because these will not change treatment in most cases. “This does not apply to situations when new symptoms develop that warrant investigation,” Dr. Ward says.
Implementation Support
Figure 1 in the guideline provides a comprehensive graphic overview of key treatment actions for patients with active AS and stable AS. The figure is designed to help practitioners determine treatment approaches according to first-, second- and third-line therapies and delineated by recommendation strength.
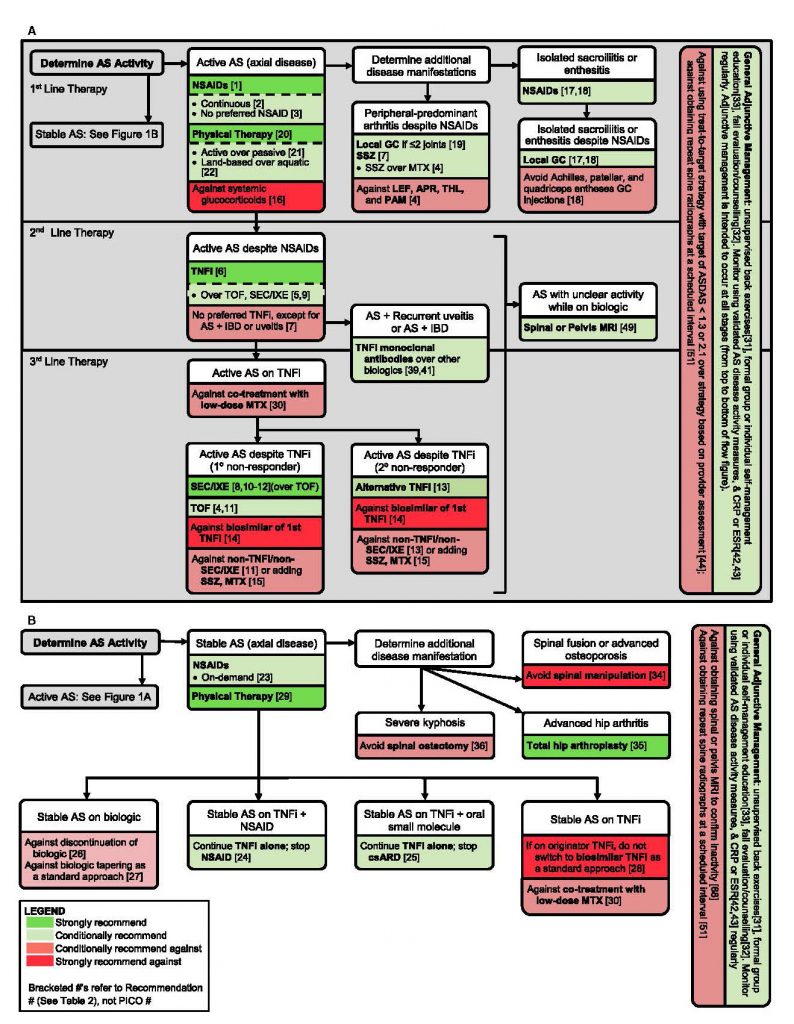
Figure 1. A comprehensive graphic overview of key treatment actions for patients with active AS and stable AS.
“We hope the figure presents an easy-to-follow capsule summary of the main recommendations,” Dr. Ward says. The ACR is also planning to develop a pocket card, as well as add the guideline to its mobile app.
“The ultimate goal in releasing this guideline update is to speed patient receipt of appropriate medications, while avoiding unnecessary imaging procedures and, ultimately, improving symptoms and health status for patients with axial SpA,” says Dr. Ward.
Carina Stanton is a freelance science journalist based in Denver.
References
- Ward MM, Deodhar A, Gensler LS, et al. 2019 Update of the American College of Rheumatology/Spondylitis Association of America/Spondylitis Association of America/Spondyloarthritis Research and Treatment Network Recommendations for the Treatment of Ankylosing Spondylitis and Nonradiographic Axial Spondyloarthritis. Arthritis Care Res (Hoboken). 2019 Aug 21. [Epub ahead of print]
- About Spondylitis. Spondylitis Association of America.
- Baeton D, Sieper J, Braun J, et al. Secukinumab, an interleukin-17A inhibitor, in ankylosing spondylitis. N Engl J Med. 2015 Dec 24;373(26):2534–2548.
- van der Heijde D, Cheng-Chung Wei J, Dougados M, et al. Ixekizumab, an interleukin-17A antagonist in the treatment of ankylosing spondylitis or radiographic axial spondyloarthritis in patients previously untreated with biological disease-modifying anti-rheumatic drugs (COAST-V): 16 week results of a phase 3 randomised, double-blind, active-controlled and placebo-controlled trial. Lancet. 2018 Dec 8;392(10163):2441–2451.