NEW YORK (Reuters Health)—The selective Janus kinase 1 and 2 inhibitor baricitinib appears also to help patients whose rheumatoid arthritis (RA) has not responded adequately to biologic disease-modifying antirheumatic drugs, according to results from the RA-BEACON randomized trial.
The previously published overall results from RA-BEACON showed that baricitinib-treated patients had significantly better functional and clinical responses at week 12 than placebo.
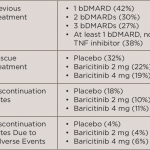
In the current study, Dr. Mark C. Genovese from Stanford University Medical Center, in Palo Alto, California, and colleagues undertook exploratory, post hoc analyses to evaluate the effect of prior use of biologic disease-modifying antirheumatic drugs (bDMARDs) on the efficacy and safety of baricitinib.
For the primary endpoint, a 20% improvement in American College of Rheumatology criteria (ACR20) at week 12, there were no significant interactions based on the patient’s prior experience by the number of prior dMARDS, the number of prior TNF inhibitors, the number of prior non-TNF inhibitor treatments, or specific prior TNF inhibitor, the team reports in Rheumatology, online February 3.
Similarly, when evaluating ACR20 responses at weeks 12 and 24 according to the patient’s last bDMARD before randomization, the researchers found that response rates for baricitinib were numerically higher than those for placebo regardless of which TNF inhibitor or non-TNF inhibitor was used, with the exception of baricitinib 2 mg versus infliximab.
There was no increase in serious infections for either baricitinib dose according to prior biologic use, including for patients who had received multiple prior biologics.
“Baricitinib demonstrated a consistent, beneficial treatment effect in bDMARD-refractory patients across subgroups based on baseline characteristics and prior bDMARD use,” the researchers conclude. “Because the population of rheumatoid arthritis patients who are TNF-inhibitor inadequate responders is increasing, this finding may be clinically relevant.”
Dr. Genovese did not respond to a request for comment.
Eli Lilly & Co. and Incyte sponsored the trial. Eli Lilly employed half of the authors and had various relationships with several others.
Reference
- Genovese MC, Kremer JM, Kartman CE, et al. Response to baricitinib based on prior biologic use in patients with refractory rheumatoid arthritis. Rheumatology (Oxford). 2018 Feb 3. [Epub ahead of print]