Soon, rheumatologists may have another drug to offer their patients with refractory rheumatoid arthritis (RRA) for whom effective and safe treatment remains challenging.
A study published in the New England Journal of Medicine shows that patients with RRA treated with once-daily baricitinib in a 4 mg dose had a significant clinical improvement in symptoms of active disease compared with placebo.1
“Baricitinib was effective in the treatment of patients with active rheumatoid arthritis,” says lead author of the study, Mark C. Genovese, MD, the James W. Raitt MD Professor, Department of Medicine, Division of Immunology & Rheumatology, Stanford University Medical School, Stanford, Calif. “Importantly, it demonstrated efficacy in a population of patients who had previously failed to adequately respond to many other currently available conventional and biologic therapies.”
The Study
In the international, multicenter, Phase 3 study, called the RA-BEACON trial, 527 patients with RRA were randomized to receive 2 mg baricitinib daily (n=174), 4 mg baricitinib daily (n=177) or placebo (n=176) for 24 weeks. All patients were at least 18 years old, had moderate to severe active RA (≥6 tender joints of 68 joints examined and serum C-reactive protein level ≥3 mg/L) and had an inadequate response to, or unacceptable side effects with, at least one prior treatment with a tumor necrosis factor (TNF) inhibitor, other biologic disease-modifying anti-rheumatic drug (bDMARD) or both. Table 1 (below) lists treatment-related characteristics.
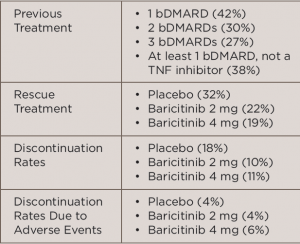
(click for larger image)
TABLE 1: Treatment-Related Characteristics
Key: bDMARD, biologic disease-modifying anti-rheumatic drugs; TNF, tumor necrosis factor
The primary endpoint of the study was the proportion of patients who had an ACR20 response at 12 weeks, with the primary comparison between patients treated with baricitinib 4 mg daily and placebo.
Based on the primary endpoint, the study found that significantly more patients treated with 4 mg baricitinib had an ACR20 response at 21 weeks compared with placebo (55% vs. 27%, P<0.001).
The study also assessed secondary endpoints, including ACR50 and ACR70 responses, physical function as measured by the Health Assessment Questionnaire-Disability Index (HAQ-DI) and disease activity as measured by the 28-joint Disease Activity Score (DAS). The last measure is based on the level of high-sensitivity C-reactive protein (DAS28-CRP) or the erythrocyte sedimentation rate (DAS28-ESR), the Clinical Disease Activity Index (CDAI) and the Simplified Disease Activity Index (SDAI).
Based on these endpoints, the study found that patients treated with 4 mg baricitinib also had significant improvements compared with placebo in DAS28-CRP and HAQ-DI (<0.001 for both measures). No significant difference between the two groups was seen for SDAI.
Results
Commenting on these results, Ziv Paz, MD, an instructor in medicine at the Beth Israel Deaconess Medical Center and Harvard Medical School, Boston, emphasized the importance of these results in treating a very challenging population of patients.
Unlike many other studies that have evaluated biologics largely in patients with RA who are naive to biologics or failed first-line treatment, such as those with early disease, he stresses the study by Genovese and colleagues is unique in that it evaluates efficacy of a new agent in a difficult-to-treat population. “We lack good interventional randomized controlled trials involving these challenging patients,” he says.
Oral JAKs
Baricitinib is the second drug in the relatively new class of drugs called oral Janus kinase (JAK) 1 and 2 inhibitors. The first drug in this class, tofacitinib, was approved by the Food and Drug Administration (FDA) in May 2012 for RRA patients.
Saying he currently uses tofacitinib for his RRA patients, Dr. Paz says the potential availability of baricitinib will provide an additional treatment option from the JAK-inhibitor class of agents for patients who do not respond well to tofacitinib. “This would be similar to the current strategy of exchanging between biologics from the same class, [such as] TNF inhibitors, to find the right match for our patients,” he says.

Dr. Genovese
Currently, baricitinib is under regulatory review by the FDA for treatment of inflammatory and autoimmune diseases. According to a press release by the manufacturer of the drug, four Phase 3 clinical trials of the drug have been conducted to “support regulatory submission in most countries.”2
“Hopefully, [these results] will translate to a commercially approved therapy to help patients with active disease,” said Dr. Genovese.
Safety
One big concern raised by the study is the adverse events reported with baricitinib, says Dr. Paz. Both groups of patients receiving baricitinib at the 2 mg and 4 mg doses had higher rates of adverse events than the placebo group (71% and 77% vs. 64%, respectively), including higher rates of infections (44% and 40% vs. 31%, respectively). Headache, upper respiratory infections and nasopharyngitis were the most common adverse events in the patients treated with baricitinib. None of the patients had opportunistic infections, tuberculosis or gastrointestinal perforations.
Rates of serious adverse events were highest in the 4 mg baricitinib group followed by the placebo group, with the fewest in the 2 mg baricitinib group (10%, 7% and 4%, respectively). In the 4 mg group, the serious adverse events included two nonmelanoma skin cancers and two major adverse cardiovascular events, which included a fatal stroke.
Emphasizing that the adverse events reported in the study are high, Dr. Paz points out the high rate of serious adverse events in the placebo group indicates the need to ask why this patient population had such a high rate of adverse events.
“We have to understand that these patients have been treated with methotrexate, steroids and other immunosuppressive medications, so they are prone to develop infections and other complications even without the addition of the biologic agent,” he says. “So it is hard to attribute all the adverse events just to the JAK inhibitor.”

Dr. Paz
Despite these high rates of adverse events, Dr. Paz says “we have limited treatment options for these patients so I would consider using this medication in patients who failed multiple other interventions and are sick and disabled.”
Saying that gathering safety data across all Phase 3 studies on baricitinib is needed to evaluate when the drug may be contraindicated for which patients, Dr. Genovese emphasizes that the study results provide hope for these difficult to treat patients.
“The message of this study should be one of optimism and hope for both patients and providers that therapies in clinical development can be effective for patients with rheumatoid arthritis and active disease,” Dr. Genovese says.
Mary Beth Nierengarten is a freelance medical journalist based in Minneapolis.
References
- Genovese MC, Kremer J, Zamani O, et al. Baricitnib in patients with refractory rheumatoid arthritis. N Engl J Med. 2016 Mar 31;374(13):1243–1252.
- Eli Lilly and Co., Incyte Corp. Phase 3 study findings demonstrate treatment with baricitinib results in significant improvements for patients with rheumatoid arthritis who had inadequate response to biologics: Pivotal RA-BEACON Study Published in New England Journal of Medicine. PR Newswire. 2016 Mar 31.


