Articular cartilage defects are common, being encountered in more than half of all knee arthroscopies.1 While not all defects are symptomatic, those that are can cause disability comparable to that seen in patients awaiting knee replacement for advanced osteoarthritis.2 Early attempts at cartilage repair tended to focus on the defect itself, often disregarding articular comorbidities, such as malalignment or meniscal deficiency that were the root cause for the development of cartilage damage in the first place. Not surprisingly, early reports of cartilage repair were disappointing; for example, Brittberg reported good outcomes in only two of seven patients with autologous chondrocyte implantation (ACI) to the patella.3 In contrast to these disappointing early results, more recent studies have shown success in more than 70% of patients treated with patellar ACI.4-6 This substantial improvement has been attributed to the increased recognition and treatment of articular comorbidities concurrently with cartilage repair in a process termed biologic joint reconstruction. While many of the surgical procedures involved are technically challenging, there is wide consensus that the most challenging aspect of this field remains in correctly identifying the appropriate surgery for each patient’s problem.
Rationale for Biologic Joint Reconstruction
Knee replacement provides good pain relief with a relatively high patient satisfaction, ranging from 73% to 85%.7,8 Joint registries and large cohorts have demonstrated 10-year survival rates of 80% to 90% for total knee replacements.9,10 However, the majority of these studies were conducted in older patients, ranging in age from the late 60s to early 80s. Younger patients are less satisfied with the outcome and also demonstrate higher implant failure rates, with a three-year revision rate of 3% reported in patients younger than 55; only 50% of patients younger than 40 demonstrated either good or excellent Knee Society function scores following a total knee replacement, and the revision rate was 12.5% at eight years.11-13 These young patients will likely require multiple revision surgeries during their lifetime and these procedures result in progressively compromised outcomes.14,15 It therefore appears reasonable to attempt delaying arthroplasty as long as possible in young patients through early intervention to normalize the biomechanical environment and repair cartilage defects that have already developed.
Diagnosis of Cartilage Defects
Symptomatic cartilage defects typically present with activity-related joint pain referable to the respective compartment (medial, lateral, or patellofemoral). Diffuse pain of the entire joint is atypical and should raise concerns for either more advanced tricompartmental osteoarthritis or other processes not generally amenable to cartilage repair. Swelling and effusion are seen especially with trochlear defects. Clicking and popping is a common, nonspecific joint complaint even in structurally sound knees, but can be associated with larger defects.
There are no symptoms pathognomonic for cartilage defects, and patients are frequently diagnosed with meniscal tears or patellofemoral syndrome initially.
Physical Findings
Similar to the patient’s presenting complaint, physical examination will not produce any findings specific for cartilage repair. Range of motion is usually preserved, although displaced osteochondral fragments can lead to intermittent locking. Large defects, especially in the patellofemoral compartment, can lead to reproducible catching or clicking with active and passive motion and patellar manipulation. Tenderness to palpation of the femoral condyles and joint spaces suggests the presence of synovitis. Given the importance of articular comorbidities, particular attention should be focused on the assessment of ligamentous stability, patellar maltracking, and alignment.
Diagnostic Imaging
Standard radiographs are utilized to screen for advanced osteoarthritic changes that would rule out cartilage repair as well as to assess the lower extremity for malalignment. Imaging includes weightbearing anteroposterior views in full extension; posterior–anterior (Rosenberg) views in mild flexion; lateral and patellofemoral views; and a full-length hip-to-ankle radiograph.
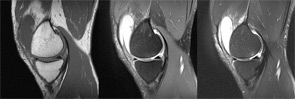
High-resolution (1.5 Tesla and greater) MRI is a reliable means to evaluate articular cartilage defects. The recent introduction of cartilage-specific imaging protocols has dramatically improved the quality of MRI scans and allows noninvasive monitoring after cartilage repair procedures (see Figure 1).16,17
Indications for Cartilage Repair
Many cartilage defects are asymptomatic; therefore, careful assessment of other potential pain generators is critical prior to consideration of surgical treatment. Nonoperative treatment with rehabilitation, activity modification, and antiinflammatory therapy should be considered for at least six months, especially for patellofemoral defects that typically respond well to a physical therapy regimen. Young patients with large defects are an exception to this recommendation, since lesion growth can occur even with symptomatic improvement from nonoperative treatment, potentially worsening the structural problem. For example, the majority of young patients experience almost complete resolution of knee pain following the removal of a loose osteochondritis dissecans (OCD) fragment; however, if the defect is left empty, 50% to 80% will develop radiographic evidence of osteoarthritis after eight to 10 years.18,19
Surgical intervention is considered after establishing that the patient’s symptoms are consistent with a full-thickness (Outerbridge Grade 3 or 4) cartilage defect, and adequate nonoperative management has failed to provide acceptable pain relief. Extensive counseling about the long recovery period after cartilage repair is important to avoid unrealistic expectations and disappointment.
Patients who smoke, are obese (BMI >35), or carry a diagnosis of inflammatory arthritis are not good candidates for cartilage repair surgery.20 Advanced degenerative changes with more than 50% joint space narrowing are considered a contraindication to cartilage repair in all but the very young who have intolerable symptoms and no other reasonable therapeutic options.
Treatment Algorithm
Once a decision has been made to proceed with cartilage repair, a surgical plan is formulated that defines not only the specific repair technique best suited to address the specific cartilage defect, but also incorporates any procedures required to remedy potential articular comorbidities. Treatment strategies for cartilage repair are based primarily on defect location and size. The two most common locations for cartilage defects are on the (medial) femoral condyle and in the patellofemoral compartment, each with their own treatment algorithms.1,21,22
Tibiofemoral Compartment
The choice of cartilage repair procedure for femoral defects is primarily dictated by defect size. Generally, the less invasive procedures of microfracture and osteochondral autograft transfer (OAT) have demonstrated acceptable outcomes only in defects smaller than approximately 3–4 cm2. Larger defects demonstrated only transient improvement in function, or increased donor site morbidity, respectively.23-26 Both microfracture and OAT consistently produce good and excellent results in 60% to 80% of patients with small lesions (<2–4 cm2) on the femoral condyles.27-30
Larger defects therefore are mostly treated with more invasive procedures such as osteochondral allograft transplantation or autologous chondrocyte implantation (ACI). While no studies have been performed to directly compare these more invasive procedures, large cohort studies have demonstrated good and excellent results in more than 70% of patients for each.3,31-35 Generally, defects limited to the articular surface are best treated with ACI, while defects with associated abnormalities of the subchondral bone should be considered for osteochondral allograft transplantation, which replaces both cartilage and the underlying bone.
Patellofemoral Compartment
The patellofemoral (PF) compartment is a difficult location for cartilage repair, and all techniques do worse here compared with the tibiofemoral compartment, due to its more complex geometry and higher shear forces. The importance of correcting adverse factors, such as patellar maltracking, is particularly important in this location.
While microfracture, OAT, and osteochondral allograft have generally good outcomes in the femoral condyles, there is a growing consensus that they should be used cautiously in the patellofemoral compartment except in very specific cases.
Surgical Procedures to Treat Cartilage Defects
Debridement and chondroplasty (removal of degenerated tissue and creation of mechanically stable cartilage with vertical shoulders) is generally performed arthroscopically. This can be the definitive treatment for incidentally found lesions, or initial treatment for symptomatic defects prior to consideration of more invasive cartilage repair procedures. Patients recover quickly after a debridement and chondroplasty, and patients can be fully weight bearing, but should allow pain and swelling to resolve prior to returning to activities.
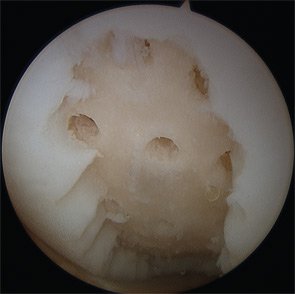
The OAT procedure involves the harvest of osteochondral plugs from lesser weight-bearing areas of the knee joint, such as the medial and lateral trochlear ridge and the intercondylar notch. The harvest and placement of the cylinders can be performed arthroscopically, but is often facilitated by a small mini-arthrotomy. Current recommendations limit this technique to the treatment of defects less than 2–3 cm2 in size; larger defects would require harvesting of a significant number of plugs, thus increasing the risk of donor site morbidity. The harvested plugs, generally 6–10 mm in diameter and 10–15 mm in length, are press-fit into the corresponding recipient site (see Figure 2). The donor site is left empty and will gradually fill with bone and fibrocartilage over time. Patients remain on protected weight bearing precautions for approximately four to six weeks with free range of motion. Athletic activities are delayed until four to six months post-op to allow for return of quadriceps strength and proprioception.
Marrow-stimulation techniques such as microfracture and subchondral drilling are arthroscopic procedures that aim to stimulate a reparative response by establishing access for bone marrow elements to the cartilage defect. The defect is cleared of any degenerated tissue, then multiple holes are created in the subchondral bone that allow blood from the marrow cavity to collect in the defect, bringing mesenchymal stem cells that induce a fibrocartilaginous repair tissue (see Figure 3). Patients remain touch-down weight bearing for six to eight weeks and utilize a continuous passive motion (CPM) machine for six hours per day for six weeks. The passive motion is thought to stimulate the generation of a higher-quality repair tissue.
Early attempts at cartilage repair tended to focus on the defect itself, often disregarding articular comorbidities, such as malalignment or meniscal deficiency that were the root cause for the development of cartilage damage in the first place. Not surprisingly, early reports of cartilage repair were disappointing.
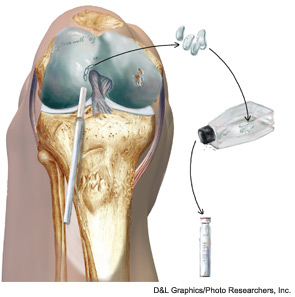
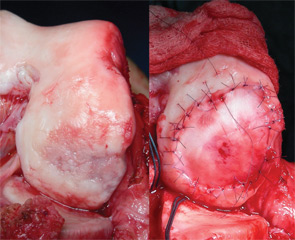
Autologous chondrocyte implantation (ACI) is currently the only cell-based therapy for cartilage repair available in the U.S., and is indicated for larger defects in the knee joint (>2–3cm2). It is a two-stage technique that requires the harvesting of a small (200–300mg) cartilage biopsy during an initial arthroscopy. The chondrocytes are released from the extracellular matrix and grown over several population doublings for approximately six weeks. This process is usually interrupted by a cryopreservation phase of up to two years to accommodate insurance approval and scheduling of the secondary reconstructive procedure. For the subsequent implantation, the defect is approached through an arthrotomy and cleared of all degenerated tissue. Originally, a thin layer of periosteum was harvested from the proximal tibia and then sutured as a patch over the defect. The use of periosteum has been largely abandoned due to the high rate at which hypertrophy occurred, which then had to be debrided with subsequent arthroscopy. Currently, the majority of ACI procedures worldwide are being performed with a collagen patch instead.36,37 Once the patch has been secured to the surrounding cartilage shoulders, the suture line is waterproofed with fibrin glue and the chondrocyte suspension is injected into the defect. The cells attach to the subchondral bone and produce matrix to form a hyaline-like tissue that slowly matures over the course of one to two years. Postoperatively, patients are generally kept on touch-down weight-bearing precautions for approximately six to eight weeks while using a CPM machine. Return to high-impact and athletic activities is usually delayed for one year.
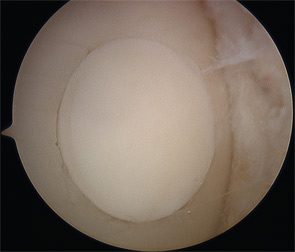
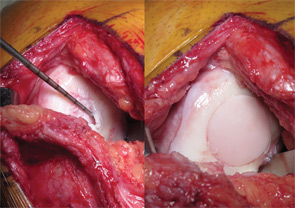
The use of osteochondral allografts is a logical extension of the OAT procedure. Similarly, cylinders of bone and cartilage are implanted to the damaged area. Due to the use of a donor source, larger grafts can be utilized than could be harvested from the patient’s own knee. Long-term studies have demonstrated survival of the transplanted chondrocytes, which appear to be immune privileged.38,39 The U.S. Food and Drug Administration and American Association of Tissue Banks (AATB) regulate the procurement, handling, and testing of donor tissue to minimize the risk of disease transmission, currently estimated at approximately 1 in 1.6 million.40 Osteochondral allograft transplantation is indicated for larger chondral and osteochondral lesions, particularly when the accompanying bone loss is greater than 6 mm, as well as a revision procedure after prior failed cartilage repair.34,35,41 Prolonged-fresh refrigerated tissue is the current standard, because freezing and freeze-drying devitalizes the tissue.40,42 Refrigerated tissue maintains up to 98% chondrocyte viability for one week, which decreases to 70% by one month.43,44 Size, side, and compartment matched grafts are preferred to facilitate restoration of the articular surface geometry. Cylindrical osteochondral plugs are most commonly used (see Figure 4), and in a majority of cases, press-fit fixation without the use of screws or pins is sufficient.35 Eventually, the bone is replaced through creeping substitution, while the articular cartilage remains allogeneic. Failure usually results from unsuccessful osseous integration and subsequent subchondral collapse.38 Rehabilitation includes touch-down weight bearing for six to 12 weeks depending on the location and size of the graft.
Articular Comorbidities Malalignment
Malalignment frequently accompanies articular cartilage defects, and has been determined as both an independent risk factor for the development (odds ratio [OR] 2.06), as well as the progression (OR 4.09) of osteoarthritis.45,46 Therefore, it stands to reason that restoration of a neutral biomechanical environment is an important factor contributing to the success of any cartilage repair procedure. Patients with a femoral condyle cartilage defect whose mechanical axis is outside the neutral zone bordered by the tibial spines should have an osteotomy.47 High tibial osteotomy (HTO) for varus malalignment and distal femoral osteotomy (DFO) for valgus malalignment are the most common methods to correct malalignment. Overcorrection with a proximal tibial osteotomy, while beneficial for advanced degenerative joint disease, is not well tolerated by the younger patients that typically experience isolated articular cartilage defects. Therefore, correction to a neutral alignment with a mechanical axis falling centrally between the tibial spines is recommended. Overcorrection by shifting the axis more into the contralateral compartment is indicated only if there are arthritic changes in the joint.
Patellar Maltracking
Patellofemoral pain and/or instability are common causes of anterior knee pain, and most of these patients respond well to nonoperative measures. A limited number will be diagnosed with symptomatic cartilage defects that require surgical intervention, and both autologous chondrocyte implantation (ACI) and osteochondral allograft have demonstrated good outcomes; however, ACI has more positive outcomes for bipolar lesions.48,49 The goal of PF procedures is to optimize the environment for the cartilage implant, and commonly include the use of osteotomy to anteromedialize the tibial tubercle, thus reducing the contact forces across the patellofemoral joint.50 Results of PF cartilage restoration done without optimization of the PF environment are generally poor, yet using the same cartilage restoration technique with attention to PF stress has better outcomes.51,52
Meniscal Deficiency
Meniscal tears are the most common surgically treated pathology of the knee joint. Recently, there has been an increased interest in the preservation of meniscal tissue through meniscal repair, rather than meniscectomy, though some tears are found to be irreparable and substantial amounts of tissue are removed. Biomechanical studies have shown that segmental loss of meniscal tissue with disruption of the circumferential hoop bundles at any location results in biomechanical loss of that entire hoop.53 For example, both segmental loss to the capsule or a posterior horn-root tear have the same biomechanical consequence as a subtotal meniscectomy. Based on in vitro studies, most cartilage restoration algorithms treat the meniscus remnant as dysfunctional if less than a 5-mm continuous intact rim is present. The effect of meniscal tissue loss is evident more rapidly in the lateral compartment, but over time the medial compartment articular cartilage begins to break down in a large percentage of patients. Meniscal allograft transplantation decreases the stresses in a compartment with meniscal loss, and studies have reported potential chondroprotective effects with slowing, but not complete cessation of chondral degeneration.54-57 The deleterious natural history of meniscectomy and the positive effects of meniscal transplantation support the addition of meniscal transplantation to articular cartilage repair whenever substantial meniscal loss has occurred. Two prospective case series have demonstrated the positive effects of performing the two techniques concomitantly.58,59
Case Example
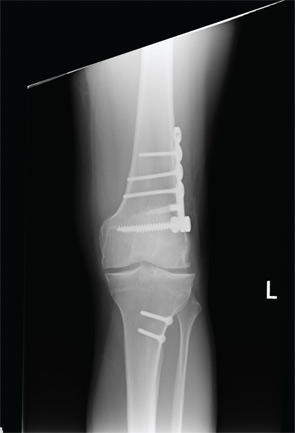
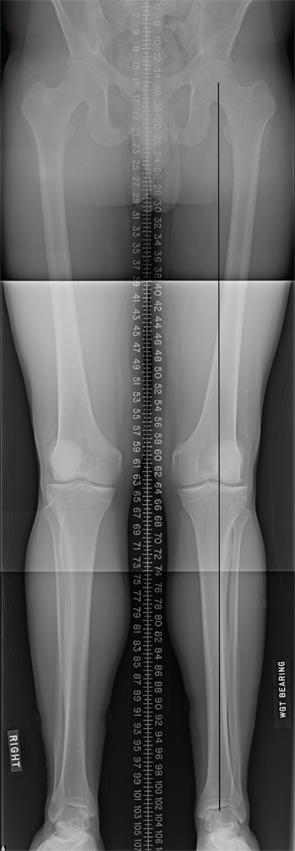
A healthy 24-year-old male presented to the office with a six-month history of persistent left lateral knee pain without recent trauma. Approximately six years previously, he had undergone arthroscopic resection of a lateral meniscal tear sustained while playing basketball. He had recovered uneventfully from surgery and return to unrestricted activities. He recently noticed increased pain and swelling, first only with impact activities, now persistently even with activities of daily living. His physical examination demonstrated a moderate effusion, mild valgus alignment of the left lower extremity, full strength, and stable ligaments. Imaging studies confirmed mild valgus alignment, minimal lateral osteophytes, and joint space narrowing (see Figure 5). An arthroscopy was performed that demonstrated subtotal lateral meniscectomy and a large chondral defect of the lateral femoral condyle. He underwent autologous chondrocyte implantation to a 10.5-cm2 defect (see Figure 6, above) in combination with lateral meniscal allograft transplantation and distal femoral osteotomy to neutralize his alignment (see Figure 7, p. 32). Postoperatively, he was kept touch-down weight bearing for eight weeks and used a CPM machine for six weeks, six hours per day. He is now two years out from surgery and is functioning well without pain or swelling with activities of daily living.
This case is a typical example of biologic joint reconstruction: a healthy joint in a young patient developed accelerated degeneration due to subtotal lateral meniscectomy. Cartilage repair alone would have addressed the defect, but not its etiology, resulting in a very high chance of early recurrence of cartilage damage.
Conclusion
Cartilage damage encompasses a wide spectrum, ranging from focal chondral defects all the way to tricompartmental osteoarthritis. While the latter is beyond the capabilities of current cartilage repair procedures, earlier stages in the disease process can be managed successfully with cartilage repair. However, careful attention is required to correctly identify and address the frequently present comorbidities involved in the degenerative disease process, such as malalignment and meniscal deficiency.
Disclosures
Dr. Gomoll is a consultant for Genzyme Biosurgery and receives book royalties from SLACK Publishing.
Dr. Gomoll is an orthopedic surgeon in the department of orthopaedic surgery cartilage repair center at Brigham and Women’s Hospital, Harvard Medical School, in Chestnut Hill, Mass. He is a consultant for Genzyme Biosurgery.
References
- Curl WW, Krome J, Gordon ES, Rushing J, Smith BP, Poehling GG. Cartilage injuries: A review of 31,516 knee arthroscopies. Arthroscopy. 1997;13:456-460.
- Heir S, Nerhus TK, Rotterud JH, et al. Focal cartilage defects in the knee impair quality of life as much as severe osteoarthritis: A comparison of knee injury and osteoarthritis outcome score in 4 patient categories scheduled for knee surgery. Am J Sports Med. 2010;38:231-237.
- Brittberg M, Lindahl A, Nilsson A, Ohlsson C, Isaksson O, Peterson L. Treatment of deep cartilage defects in the knee with autologous chondrocyte transplantation. N Engl J Med. 1994;331:889-895.
- Minas T, Bryant T. The role of autologous chondrocyte implantation in the patellofemoral joint. Clin Orthop Relat Res. 2005;(436):30-39.
- Farr J. Autologous chondrocyte implantation improves patellofemoral cartilage treatment outcomes. Clin Orthop Relat Res. 2007;463:187-194.
- Henderson IJ, Lavigne P. Periosteal autologous chondrocyte implantation for patellar chondral defect in patients with normal and abnormal patellar tracking. Knee. 2006;13:274-279.
- Bullens PH, van Loon CJ, de Waal Malefijt MC, Laan RF, Veth RP. Patient satisfaction after total knee arthroplasty: A comparison between subjective and objective outcome assessments. J Arthroplasty. 2001;16:740-747.
- Hawker G, Wright J, Coyte P, et al. Health-related quality of life after knee replacement. J Bone Joint Surg Am. 1998;80:163-173.
- Koskinen E, Eskelinen A, Paavolainen P, Pulkkinen P, Remes V. Comparison of survival and cost-effectiveness between unicondylar arthroplasty and total knee arthroplasty in patients with primary osteoarthritis: A follow-up study of 50,493 knee replacements from the Finnish Arthroplasty Register. Acta Orthopaedica. 2008;79:499-507.
- Gioe TJ, Novak C, Sinner P, Ma W, Mehle S. Knee arthroplasty in the young patient: Survival in a community registry. Clin Orthop Relat Res. 2007;464:83-87.
- Vazquez-Vela Johnson G, Worland RL, Keenan J, Norambuena N. Patient demographics as a predictor of the ten-year survival rate in primary total knee replacement. J Bone Joint Surg Br. 2003;85:52-56.
- Sibanda N, Copley LP, Lewsey JD, et al. Revision rates after primary hip and knee replacement in England between 2003 and 2006. PLoS medicine. 2008;5:e179.
- Lonner JH, Hershman S, Mont M, Lotke PA. Total knee arthroplasty in patients 40 years of age and younger with osteoarthritis. Clin Orthop Relat Res. 2000:85-90.
- Rand JA, Trousdale RT, Ilstrup DM, Harmsen WS. Factors affecting the durability of primary total knee prostheses. J Bone Joint Surg Am. 2003;85:259-265.
- Deehan DJ, Murray JD, Birdsall PD, Pinder IM. Quality of life after knee revision arthroplasty. Acta Orthopaedica. 2006;77:761-766.
- Rosenberg TD, Paulos LE, Parker RD, Coward DB, Scott SM. The forty-five-degree posteroanterior flexion weight-bearing radiograph of the knee. J Bone Joint Surg Am. 1988;70:1479-1483.
- Potter HG, Chong le R. Magnetic resonance imaging assessment of chondral lesions and repair. J Bone Joint Surg Am. 2009;91(Suppl 1):126-131.
- Murray JR, Chitnavis J, Dixon P, et al. Osteochondritis dissecans of the knee: Long-term clinical outcome following arthroscopic debridement. Knee. 2007;14:94-98.
- Wright RW, McLean M, Matava MJ, Shively RA. Osteochondritis dissecans of the knee: Long-term results of excision of the fragment. Clin Orthop Relat Res. 2004:239-243.
- Jaiswal PK, Macmull S, Bentley G, Carrington RW, Skinner JA, Briggs TW. Does smoking influence outcome after autologous chondrocyte implantation? A case-controlled study. J Bone Joint Surg Br. 2009;91:1575-1578.
- Kreuz PC, Steinwachs MR, Erggelet C, et al. Results after microfracture of full-thickness chondral defects in different compartments in the knee. Osteoarthritis Cartilage. 2006;14:1119-1125.
- Bentley G, Biant LC, Carrington RW, et al. A prospective, randomised comparison of autologous chondrocyte implantation versus mosaicplasty for osteochondral defects in the knee. J Bone Joint Surg Br. 2003;85:223-230.
- Knutsen G, Engebretsen L, Ludvigsen TC, et al. Autologous chondrocyte implantation compared with microfracture in the knee. A randomized trial. J Bone Joint Surg Am. 2004;86-A:455-464.
- Asik M, Ciftci F, Sen C, Erdil M, Atalar A. The microfracture technique for the treatment of full-thickness articular cartilage lesions of the knee: Midterm results. Arthroscopy. 2008;24:1214-1220.
- Hangody L, Dobos J, Balo E, Panics G, Hangody LR, Berkes I. Clinical experiences with autologous osteochondral mosaicplasty in an athletic population: A 17-year prospective multicenter study. Am J Sports Med. 2010;38:1125-1133.
- Mithoefer K, McAdams T, Williams RJ, Kreuz PC, Mandelbaum BR. Clinical efficacy of the microfracture technique for articular cartilage repair in the knee: An evidence-based systematic analysis. Am J Sports Med. 2009;37:2053-2063.
- Mithoefer K, Williams RJ, 3rd, Warren RF, Wickiewicz TL, Marx RG. High-impact athletics after knee articular cartilage repair: A prospective evaluation of the microfracture technique. Am J Sports Med. Sep 2006;34:1413-1418.
- Steadman JR, Briggs KK, Rodrigo JJ, Kocher MS, Gill TJ, Rodkey WG. Outcomes of microfracture for traumatic chondral defects of the knee: Average 11-year follow-up. Arthroscopy. 2003;19:477-484.
- Gudas R, Kalesinskas RJ, Kimtys V, et al. A prospective randomized clinical study of mosaic osteochondral autologous transplantation versus microfracture for the treatment of osteochondral defects in the knee joint in young athletes. Arthroscopy. 2005;21:1066-1075.
- Hangody L, Fules P. Autologous osteochondral mosaicplasty for the treatment of full-thickness defects of weight-bearing joints: Ten years of experimental and clinical experience. J Bone Joint Surg Am. 2003;85-A(Suppl 2):25-32.
- Bartlett W, Skinner JA, Gooding CR, et al. Autologous chondrocyte implantation versus matrix-induced autologous chondrocyte implantation for osteochondral defects of the knee: A prospective, randomised study. J Bone Joint Surg Br. 2005;87:640-645.
- Peterson L, Minas T, Brittberg M, Nilsson A, Sjogren-Jansson E, Lindahl A. Two- to 9-year outcome after autologous chondrocyte transplantation of the knee. Clin Orthop Relat Res. 2000:212-234.
- Shasha N, Aubin PP, Cheah HK, Davis AM, Agnidis Z, Gross AE. Long-term clinical experience with fresh osteochondral allografts for articular knee defects in high demand patients. Cell Tissue Bank. 2002;3:175-182.
- Gortz S, Bugbee WD. Allografts in articular cartilage repair. J Bone Joint Surg Am. 2006;88:1374-1384.
- McCulloch PC, Kang RW, Sobhy MH, Hayden JK, Cole BJ. Prospective evaluation of prolonged fresh osteochondral allograft transplantation of the femoral condyle: Minimum 2-year follow-up. Am J Sports Med. 2007;35:411-420.
- Gooding CR, Bartlett W, Bentley G, Skinner JA, Carrington R, Flanagan A. A prospective, randomised study comparing two techniques of autologous chondrocyte implantation for osteochondral defects in the knee: Periosteum covered versus type I/III collagen covered. Knee. 2006;13:203-210.
- Gomoll AH, Probst C, Farr J, Cole BJ, Minas T. Use of a type I/III bilayer collagen membrane decreases reoperation rates for symptomatic hypertrophy after autologous chondrocyte implantation. Am J Sports Med. 2009;37:20S-23S.
- Kandel RA, Gross AE, Ganel A, McDermott AG, Langer F, Pritzker KP. Histopathology of failed osteoarticular shell allografts. Clin Orthop Relat Res. 1985(197):103-110.
- Williams SK, Amiel D, Ball ST, et al. Analysis of cartilage tissue on a cellular level in fresh osteochondral allograft retrievals. Am J Sports Med. 2007;35:2022-2032.
- Mroz TE, Joyce MJ, Steinmetz MP, Lieberman IH, Wang JC. Musculoskeletal allograft risks and recalls in the United States. J Am Acad Orthop Surg. 2008;16:559-565.
- Emmerson BC, Gortz S, Jamali AA, Chung C, Amiel D, Bugbee WD. Fresh osteochondral allografting in the treatment of osteochondritis dissecans of the femoral condyle. Am J Sports Med. 2007;35:907-914.
- Meyers MH, Akeson W, Convery FR. Resurfacing of the knee with fresh osteochondral allograft. J Bone Joint Surg Am. 1989;71:704-713.
- Pearsall AW 4th, Tucker JA, Hester RB, Heitman RJ. Chondrocyte viability in refrigerated osteochondral allografts used for transplantation within the knee. Am J Sports Med. 2004;32:125-131.
- Williams SK, Amiel D, Ball ST, et al. Prolonged storage effects on the articular cartilage of fresh human osteochondral allografts. J Bone Joint Surg Am. 2003;85-A:2111-2120.
- Sharma L, Song J, Felson DT, Cahue S, Shamiyeh E, Dunlop DD. The role of knee alignment in disease progression and functional decline in knee osteoarthritis. JAMA. 2001;286:188-195.
- Brouwer GM, van Tol AW, Bergink AP, et al. Association between valgus and varus alignment and the development and progression of radiographic osteoarthritis of the knee. Arthritis Rheum. 2007;56:1204-1211.
- Wright JM, Crockett HC, Slawski DP, Madsen MW, Windsor RE. High tibial osteotomy. J Am Acad Orthop Surg. 2005;13:279-289.
- Farr J. Autologous chondrocyte implantation improves patellofemoral cartilage treatment outcomes. Clin Orthop. 2007;463:187-194.
- Shasha N, Aubin P, Cheah H, Davis A, Agnidis Z. Long-term clincal experience with fresh osteochondral allografts for articular knee defects in high demand patients. Cell Tissue Bank. 2002;3:175-182.
- Beck PR, Thomas AL, Farr J, Lewis PB, Cole BJ. Trochlear contact pressures after anteromedialization of the tibial tubercle. Am J Sports Med. 2005;33:1710-1715.
- Minas T, Bryant T. The role of autologous chondrocyte implantation in the patellofemoral joint. Clin Orthop. 2005;436:30-39.
- Henderson I, Lavigne P. Periosteal autologous chondrocyte implantation for patellar chondral defect in patients with normal and abnormal patellar tracking. Knee. 2006;13:274-279.
- Jones R, Keene G, Learmouth D, et al. Direct measurement of hoop strains in the intact and torn human medial meniscus. Clin Biomech. 1996;11:295-300.
- Paletta Jr. G, Manning T, Snell E, Parker R, Bergfeld R. The effect of allograft meniscal replacement on intraarticular contact area and pressures in the human knee. Am J Sports Med. 1997;25:692-698.
- Verma N, Kolb E, Cole B, et al. The effects of medial meniscal transplantation techniques on intra-articular contact pressures. J Knee Surg. 2008;21:20-26.
- Verdonk PC, Verstraete KL, Almqvist KF, et al. Meniscal allograft transplantation: Long-term clinical results with radiological and magnetic resonance imaging correlations. Knee Surg Sports Traumatol Arthrosc. 2006;14:694-706.
- Szomor ZL, Martin TE, Bonar F, Murrell GA. The protective effects of meniscal transplantation on cartilage. An experimental study in sheep. J Bone Joint Surg Am. 2000;82:80-88.
- Farr J, Rawal A, Marberry K. Concomitant meniscal allograft transplantation and autologous chondrocyte implantation: Minimum 2-year follow-up. Am J Sports Med. 2007;35:1459-1466.
- Rue J, Yanke A, Busam M, McNickle A, Cole B. Prospective evaluation of concurrent meniscus transplantation and articular cartilage repair: Minimum 2-year follow-up. Am J Sports Med. 2008;36:1770-1778.