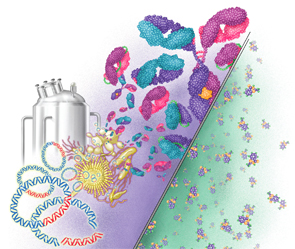
This illustration depicts the differences between biosimilars and chemically constructed small molecule drugs. Biosimilars are much bigger and more complex than small-molecule drugs.
DNA Illustrations/ScienceSource.com
Soon, biosimilars will be available as treatment options for our patients. Biosimilars are being introduced to the U.S. market in the hope that they will spur competition and drive down the price of these expensive medicines. Previous articles in The Rheumatologist have touched on various issues surrounding these new therapies, and everyone in the rheumatology community has questions about how they will fit into our armamentarium moving forward.
The Biologics Price Competition and Innovation Act of 2009 established an expedited approval pathway for biosimilars and paved the way for pharmaceutical companies to invest in and develop these agents. This past year, the FDA approved the U.S.’s first biosimilar product, filgrastim-sndz (Zarxio). On Feb. 9, 2016, the FDA’s Arthritis Advisory Committee recommended approval of an infliximab biosimilar, and on April 5, 2016, infliximab-dyyb (Inflectra) was approved for seven indications in the U.S.
First and foremost, be assured that the College is taking a strong, proactive approach to issues surrounding biosimilars. Several ACR committees are making biosimilars a top priority in 2016. The committees on Rheumatologic Care, Government Affairs and Corporate Relations, as well as the Affiliate Society Council and the Insurance Subcommittee, are regularly exchanging ideas and formulating action steps to protect clinicians and our patients as biosimilars become available. Rheumatologists are all too aware of the rising costs of prescription drugs and the struggles that we and our patients face to secure coverage from payers. Providing affordable therapeutic options is an essential part of Advancing Rheumatology! However, the ACR feels strongly that patient safety and patient and provider choice must drive upcoming decisions that the FDA will make. The ACR’s position paper on biosimilars was just updated in February.
Angus Worthing, MD, a rheumatologist in Washington, D.C., and a member of our Government Affairs Committee, shared the ACR’s position on regulatory aspects of biosimilars at the Feb. 9 FDA Arthritis Advisory Committee meeting and at a Dec. 18, 2015, Public Meeting on the Reauthorization of the Biosimilar User Fee Act (BsUFA). The ACR:
- Supports the Reauthorization of the Biosimilar User Fee Act (BsUFA);
- Holds that biosimilars must have distinct names, allowing them to be distinguished from each other and their reference products;
- Asserts that packaging language must a) indicate whether a biosimilar is interchangeable with the reference (originator) biologic, b) state all indications for which a biosimilar is approved and c) specify whether the supporting clinical data for the indication are derived from studies of the biosimilar or the reference product;
- Supports a requirement for clinical data to ensure biosimilars’ safety and efficacy prior to approval, not just extrapolation of data derived from studies of the reference biologic; and
- Supports a requirement that long-term, post-marketing studies of approved therapies be conducted to monitor for adverse events.
Several unanswered questions about biosimilars continue to be debated by the FDA and other stakeholders. In the coming weeks, we may know more about how prescribers will be notified when their prescriptions are filled by the pharmacy, what role payers will play in deciding drug substitutions and how biosimilars will be tracked for post-market efficacy and safety. That’s why the ACR must take an active role in driving this discussion. We want our voices to be heard, and we want to advocate for positions that will be beneficial for you and your patients. Now is the time for rheumatologists to speak up and speak out about biosimilars.



