Osteoporosis is a disease defined by low bone strength such that the bone fractures when exposed to unusually low stress. While many aspects of osteoporosis are not yet understood, research during the past 10 years has provided important information to identify individuals at high risk for the disease, institute screening, and offer treatment for patients who are at risk of fracture. We now have several effective bone-active agents that improve bone strength and reduce incident fractures in individuals at high risk for fracture. More effective bone-active agents in development may soon be approved.
While the data to support the screening and medical treatment of this disease are strong, management of osteoporosis in clinical practice is complicated by uncertainty in applying evidence from clinical trials to the care of individual patients. Compared with patients enrolled in trials, patients in clinical practice may have more comorbidities, less understanding of the disease, less motivation to adhere to the prescribed therapy, and financial concerns that—combined—can reduce the efficacy of the prescribed bone-strengthening agent.
In light of the challenges the clinician faces in diagnosing and treating osteoporosis, this article will review current osteoporosis screening techniques, approved medications and their optimal duration of use, sequential therapy, and secondary causes of osteoporosis that the rheumatologist may encounter.
Screening for Osteoporosis
In 1992, the World Health Organization (WHO) adopted a method for defining osteoporosis by using measurements of bone mineral density (BMD) obtained from dual energy X-ray absorptiometry (DXA) machines. The T score is defined as the number of standard deviations above or below peak bone mass obtained at about age 20. A T score of greater than -1.0 is normal, -2.5 to -1.0 is osteopenia, and below -2.5 is osteoporosis. The T scores, which were developed to assess the prevalence of low bone mass in the population, were not intended to identify subjects who might benefit from treatment with bone active agents. However, in randomized, controlled clinical trials that evaluated the efficacy of osteoporosis agents, women with T scores below -2.5 were enrolled to determine if agents could reduce the risk of new vertebral fractures.
A number of subspecialty societies—including the National Osteoporosis Foundation (NOF) and the Endocrine Society, among others—recommended that physicians and other healthcare providers offer treatment to prevent osteoporotic fractures if the patient had a T score less than -1.5 with risk factors, and less than -2.0 without risk factors. Clinicians have followed these recommendations, screening patients for osteoporosis and making treatment recommendations based on the BMD results. A number of new developments have made clinicians and policymakers question these recommendations. First, patients’ adherence to osteoporosis treatment if they have not experienced a fracture is surprisingly low. Second, a number of women and men who have T scores above the osteoporotic level can have fractures.
These and other issues have motivated a group of epidemiologists and clinicians who treat patients for osteoporosis to develop a new set of WHO recommendations for preventing osteoporotic fractures. The final document is not yet ready for dissemination. However, these new recommendations combine clinical risk factors that increase osteoporotic fracture risk (see Table 1) and a bone mineral density measurement—if available—to provide a 10-year risk of a hip fracture. The risk threshold used to recommend treatment may differ by country but, for the United States, hip fracture prevention treatment will be recommended if the 10-year risk is about 3%.1
Therapies: Anti-Resorptive Agents
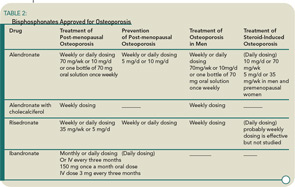
Medications for the prevention and treatment of osteoporosis currently include anti-resorptive agents (bisphosphonates, selective estrogen receptor modulators, calcitonin, and estrogen) and one anabolic agent (rhPTH 1-34 or teriparatide). (See Table 2) Physicians generally initiate therapy for patients with osteoporosis with a bisphosphonate; if the patient suffers fractures or is intolerant to the oral or intravenous bisphosphonates, rhPTH 1-34 is prescribed. Because osteoporosis is a chronic disease, physicians usually initiate treatment for patients who are around age 60 and will continue to monitor the patients bone status for another 20 to 25 years. Like patients with any other chronic disease who take a medication for a long time, osteoporosis patients ask their physicians if they need to continue the medication. Generally this happens after five or more years of weekly therapy with a bisphosphonate. This is a difficult question to answer because the clinical trials performed for FDA approval only last for three years, and information on fracture reduction after that period is not available for most of the osteoporosis agents now in use.
An extension study, Fracture Intervention Trial Long-Term Extension (FLEX), was recently published. This study addressed the duration of therapy and evaluated patients enrolled in the Fracture Intervention Study (FIT) who had been on alendronate for three years. These patients had a T score of the total hip or femoral neck of greater than -3.0 (higher than the mean at entry into the FIT trial). Nearly 1,000 women were randomized to placebo, 5 mg of alendronate a day, or 10 mg of alendronate a day for three years. Study endpoints included bone mineral density, biochemical markers of bone turnover, and incident vertebral and nonvertebral fractures.
Although study subjects were off medication for between one and two years, those randomized to the placebo lost about 2% of hip bone mass but had no decline in lumbar spine bone mass. They also had increasing CTX-1 (a marker of osteoclast activity) but no increase in morphometric vertebral fractures or nonvertebral fractures compared with the alendronate-treated subjects.2 These findings suggest that, in patients who have been treated with a bisphosphonates for more than three years, it is probably safe to discontinue the medication if they have responded well, have a T score of the total hip or femoral neck of greater than -2.5, have not experienced a low-impact fracture while on the medication, and have low risk of an osteoporotic fracture.
If a bisphophonate is discontinued, the patient will need to be rescanned by hip DXA within two to four years, depending on the patient and overall risk of an osteoporotic fracture. It may also be useful to measure a biochemical marker of bone turnover such as urine N-telopeptide or serum C-telopeptide. Test for bone turnover when the patient stops the bisphosphonate medication and annually thereafter. If the marker increases from a premenopausal low-turnover rate to a higher postmenopausal turnover rate, it might be appropriate to obtain another hip BMD and consider reinstituting the therapy.
In deciding therapy duration, it is important that any patient who has suffered a low-impact fracture while on anti-resorptive treatment continue therapy. Almost none of the bone-active agents we use today for the treatment of osteoporosis cure the disease, but they do reduce the risk of fractures in most cases by 50% to 60%. Patients can still have fractures while on these medications, although they may have one fracture rather than two.
Safety and Selection
The safety of long-term bisphosphonates appears good. The most common adverse effects are gastrointestinal, including esophagitis, heartburn, abdominal pain, and diarrhea, but the length of use does not appear to change an individual’s risk for these side effects. Also, some patients experience acute adverse events, including fever and myalgias, with intravenous bisphosphonates and occasionally with oral bisphosphonates. But these events tend to be short lived and less severe with continued use of the medications.
There is not much data to support the use of one bisphosphonate over another. A study funded by Merck compared BMD and biochemical markers of bone turnover in postmenopausal women with osteopenia who were randomized to either alendronate 70 mg a week or risedronate 35 mg a week.3 Alendronate increased BMD of the spine and hip slightly more than risedronate. However, because all anti-resorptive agents have anti-fracture effects that are greater than their change in BMD, without fracture data it is not possible to make claim for a difference between the two anti-resorptive agents.
Another study—the risedronate and alendronate (REAL) cohort study—evaluated a large number of subjects from a health claims database, who were treated with either alendronate (n=21,615) or risedronate (n=12,215). The study endpoint was new fractures. The study reported that risedronate was more effective than alendronate for anti-fracture efficacy.4 There were 507 nonvertebral fractures and 109 hip fractures. Through one year of therapy, the incidence of nonvertebral fractures in the risedronate cohort (2.0%) was 18% lower (95% confidence interval [CI] 2%–32%) than in the alendronate cohort (2.3%). The incidence of hip fractures in the risedronate cohort (0.4%) was 43% lower (95% CI 13%–63%) than in the alendronate cohort (0.6%). These results were consistent across a number of sensitivity analyses such that, given the shortcomings of using a medical utilization database, they are probably valid.
Recently, there have been reports of osteonecrosis of the jaw (ONJ) associated with bisphosphonate use—especially intravenous bisphosphonates and in patients with metastatic cancer. ONJ is an area of exposed bone in either the mandible or the maxilla that is slow to heal over a number of months. A few epidemiologic studies have identified factors for this condition that include a history of periodontitis, dental surgery, underlying malignancy, greater than one and a half years of intravenous bisphosphonate use, and poor dental hygiene.
While ONJ is a rare complication, encourage patients treated with bisphosphonates to have a dental examination before initiating a bisphosponate and at regular intervals.5 Because bisphosphonates have a long half-life in bone, there are no data to support discontinuation of the medication prior to a dental procedure to reduce the risk of ONJ.
Therapies: Anabolic Agents
Teriparatide (PTH), which is approved for the treatment of osteoporosis, differs from the anti-resorptive agents in its actions. PTH is an anabolic agent that stimulates osteoblasts to form new bone while simultaneously increasing osteoclastic bone resorption through increasing receptor activator for nuclear factor k B ligand factor (RANKL) production. While bone turnover is increased with PTH, overall bone mass and bone strength increase with this agent, reducing fracture risk. While PTH is approved for the treatment of osteoporosis, it is generally not used as a first-line agent because of cost and the need for injections.
Initially, clinicians questioned whether combined treatment with an anti-resorptive agent and PTH could increase bone mass faster than treatment with the anabolic agent alone by blocking bone remodeling. Black et al. determined that concurrent treatment with PTH and alendronate was not more effective than PTH alone.6 PTH stimulates new bone formation and, by increasing the production of RANKL, also bone remodeling. Increased bone remodeling releases growth factors stored in the bone matrix to further stimulate new bone formation. Bisphosphonates block the bone remodeling and release of growth factors stored in the bone matrix. However, treatment with PTH should be followed by a bisphosphonate; this allows the remodeling space to fill in and fully mineralize. Patients who do not initiate anti-resorptive treatment after a one and a half- to two-year course of PTH will slowly lose the new bone mass gained.7
While PTH can build new bone and improve bone strength, we do not know the optimal treatment duration or dose. We also do not know if prior use of a bisphosphonate blunts the effect of PTH on bone mass and strength. Over the next few years, expect to see other formulations of PTH; different modes of delivery are currently being tested. A strategy to form new bone with PTH and build up bone strength and then maintain it with a potent anti-resorptive agent may go a long way toward reducing osteoporotic fracture risk.
Glucocorticoid-Induced Osteoporosis
Glucocorticoid-induced osteoporosis (GIOP) is an unfortunate complication of glucocorticoid use. The mechanism by which glucocorticoids increase fracture risk is still under debate. Initially, glucocorticoids appear to increase osteoclast maturation and function, although this is followed by a reduction in osteoblast and osteocyte activity and lifespan. Therefore bone mass, especially trabecular bone mass, is reduced rapidly with glucocorticoid use. In addition, glucocorticoids decrease calcium absorption in the gastrointestinal tract and increase excretion from the kidney, which can create a negative calcium balance and further increase bone remodeling.8 Prevent GIOP through adequate calcium and vitamin D supplements to augment calcium absorption in the gastrointestinal tract. In addition, nearly all bisphophonates prevent and treat glucocorticoid-induced bone loss by inhibiting osteoclast activity.
In vitro and animal studies of GIOP indicate that glucocorticoids reduce mineralization gene expression, and bisphosphonates appear to prevent suppression of mineralization genes by osteocytes and reduce osteocyte apoptosis. In addition to reducing osteoclast-mediated bone remodeling, bisphosphonates may prevent changes in the osteocyte induced by glucocorticoids, helping to maintain bone strength.
One of the main changes in bone metabolism that occur in the presence of glucocorticoids is reduced bone formation. Nearly 10 years ago, studies by our research group found that treating women with postmenopausal osteoporosis and GIOP using PTH could override the glucocorticoid-induced suppression of bone formation and increase bone mass.9 It might be worthwhile to encourage patients treated with glucocorticoids who have osteoporosis to initiate PTH treatment to build up their bone mass and then maintain it with a bisphosphonate.
Inflammatory Bone Loss
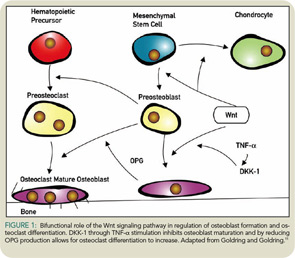
As rheumatologists, we frequently see bone loss adjacent to joints that have inflammation. Patients with RA have both bone erosions and reduced bone mass around the erosion. Recently, investigators have shown that a new signaling pathway, the Wnt/b-catenin signaling pathway, which is important in skeletal development and repair, is involved in these bone changes around joints with inflammation. (See “Reading Rheum,” May 2007) The Wnt pathway has agonists that increase signaling through the frizzled receptor, which then transports a protein—b-catenin—into the nucleus to signals bone cells to mature (see Figure 1).
The Wnt signaling pathway also has antagonists that stop bone formation; one is Dickkopf-1 (DKK-1). In the presence of synovial inflammation, fibroblast-like synoviocytes stimulated by tumor necrosis factor (TNF)-a produce DKK-1, which then stops new bone formation around the area of inflammation. At the same time, DKK-1 reduces the production in preosteoblasts of OPG, so that osteoclasts are stimulated to mature and resorb bone. Therefore, with synovial inflammation like that which occurs in RA, DKK-1 production may reduce bone formation and increase bone resorption. Treatment of TNF transgenic mice with anti–DKK-1 and an anti-TNF blocking agent reduced joint destruction.10,11 Interestingly, patients with active RA have also been shown to have increased serum levels of DKK-1.
Because TNF stimulates DKK-1 production, anti-TNF agents used to treat RA patients with active joint inflammation may reduce TNF-a and, most likely, DKK-1. As a result, patients may have less structural deterioration and possibly some healing of bone loss. The investigations linking the Wnt signaling pathway with bone and joint destruction in RA are fascinating, reminding us that the most important way to prevent structural deterioration in our patients is by reducing joint inflammation. We can accomplish this goal with aggressive anti-TNF blocker use, which can improve bone mass structure around the inflamed joints and also change the biochemical markers of bone turnover toward formation rather than resorption.
Because bone resorption is increased in patients with RA, investigators have always wondered if a potent anti-resorptive agent could reduce the development of new bone erosions. Recently, two studies addressed these questions.
In the first, patients with active RA were randomized to intravenous zoledronic acid or placebo. After six months, patients treated with the bisphosphonate has a significant reduction in new erosions as assessed by MRI.12
In the second study, patients with active RA taking methotrexate were randomized to placebo (60 mg) or 180 mg of a monoclonal RANKL inhibitor (denosumab). Denosumab use reduced new bone erosions seen on MRI at six months and significantly reduced new erosions shown on radiograph after 12 months.13 Potent reduction in osteoclast activity caused by new agents may prevent structural deterioration in RA patients. However, neither bone active agent reduces joint inflammation (the main source of the osteoclasts that create erosions). At present, the most important treatment strategy for erosion prevention targets joint inflammation because data indicate that TNF blocking agents that reduce joint inflammation can also reduce new joint erosions very effectively. Future studies should provide new information on the effect bone-active agents have on both local and generalized both loss in inflammatory disease and whether these agents can promote healing as well as prevent damage.
Conclusion
During the last 10 years, clinicians have been able to identify patients at risk for osteoporotic fractures. Potent anti-catabolic agents and anabolic agents—either used alone or sequentially—are effective in preventing incident fractures and treating established disease for both postmenopausal osteoporosis and glucocorticoid-induced osteoporosis.
However, we still have challenges ahead in the field of osteoporosis. We need more studies to further our understanding of how current bone-active agents improve bone strength, how long to treat patients with bone-active agents to improve bone strength, and how to better identify patients who will benefit from fracture prevention treatment. Although it is a chronic degenerative disease, osteoporosis can be diagnosed and treated, and—with optimal medical management—hopefully it can be cured.
References
- Kanis JA, Oden A, Johnell O, et al. The use of clinical risk factors enhances the performance of BMD in the prediction of hip and osteoporotic fractures in men and women. Osteoporos Int. 2007; E-pub ahead of print: DOI 10.1007/s00198-007-0343-y.
- Black DM, Schwartz AV, Ensrud KE, et al. Effects of continuing or stopping alendronate after five years of treatment: the Fracture Intervention Trial Long-term Extension (FLEX): a randomized trial. JAMA. 2006;296(24):2927-2938.
- Rosen CJ, Hochberg MC, Bonnick SL, et al. Treatment with once-weekly alendronate 70 mg compared with once-weekly risedronate 35 mg in women with postmenopausal osteoporosis: a randomized double-blind study. J Bone Miner Res. 2005;20(1):141-151.
- Silverman SL, Watts NB, Delmas PD, Lange JL, Lindsay R. Effectiveness of bisphosphonates on nonvertebral and hip fractures in the first year of therapy: the risedronate and alendronate (REAL) cohort study. Osteoporos Int. 2007;18(1):25-34.
- Woo SB, Hellstein JW, Kalmar JR. Narrative [corrected] review: bisphosphonates and osteonecrosis of the jaws. Ann Intern Med. 2006;144(10):753-761.
- Black DM, Greenspan SL, Ensrud KE, et al. The effects of parathyroid hormone and alendronate alone or in combination in postmenopausal osteoporosis. N Engl J Med. 2003;349(13):1207-1215.
- Black DM, Bilezikian JP, Ensrud KE, et al. One year of alendronate after one year of parathyroid hormone (1-84) for osteoporosis. N Engl J Med. 2005 Aug 11;353(6):555-565.
- van Staa TP. The pathogenesis, epidemiology and management of glucocorticoid-induced osteoporosis. Calcif Tissue Int. 2006;79(3):129-137.
- Lane NE, Sanchez S, Modin G, et al. Parathyroid hormone treatment can reverse steroid osteoporosis: results of a randomized clinical trial. J Clin Invest. 1998;102(8):1627–1633.
- Diarra D, Stolina M, Polzer K, et al. Dickkopf-1 is a master regulator of joint remodeling. Nat Med. 2007; 13(2):156–163.
- Goldring SR, Goldring MB. Eating bone or adding it: the Wnt pathway decides. Nat Med. 2007;13(2):133-134.
- Jarrett SJ, Conaghan PG, Sloan VS, et al. Preliminary evidence for a structural benefit of the new bisphosphonate zoledronic acid in early rheumatoid arthritis. Arthritis Rheum. 2006;54(5):1410-1414.
- Cohen SB, Dore RK, Lane N, et al. Denosumab treatment effects on structural damage, bone mineral density and bone turnover in rheumatoid arthritis: A randomized, double-blind, placebo-controlled clinical trial. NEJM, in press.
Hospitalists as Test Subjects

Dr. Lane is endowed professor of medicine, vice chair for research in the department of medicine and rheumatology, and director at the Center for Healthy Aging at the University of California at Davis (UCD) in Sacramento. She received her undergraduate degree with highest honors at UCD and her medical degree at the University of California at San Francisco. Dr. Lane completed a residency in medicine at Mount Zion Hospital and Medical Center in San Francisco and a rheumatology clinical fellowship at the Palo Alto VA Hospital and Stanford University Medical Center (Calif).
Dr. Lane’s research focuses on bone biology and the epidemiology of osteoarthritis of the hip. She is currently evaluating how bone-active agents alter osteoblast and osteoclast activity and influence bone material properties. She is a principal and co-principal investigator of several clinical trials focusing on osteoporosis, protection of the skeleton from glucocorticoid-induced bone loss, and investigations into the unique aspects of osteobiology.
Dr. Lane belongs to many professional organizations, including the ACR, American Society of Bone and Mineral Research, Orthopedic Research Society, and Osteoarthritis and Cartilage Research Society. She has been the president of the U.S. Bone and Joint Decade executive board since 2005. She is co-editor of Arthritis and Rheumatism, associate editor of Primer of Metabolic Bone Diseases, and is on the editorial board of several other journals. She has authored or co-authored over 130 articles.
Dr. Yao is assistant adjunct professor at the UCD Center for Healthy Aging.