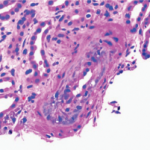
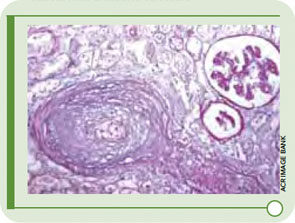
How does one study rare events in uncommon diseases? At a meeting of the Scleroderma Clinical Trial Consortium a few years ago, a heated debate broke out about the role of angiotensin-converting enzyme (ACE) inhibitors as prophylaxis for scleroderma renal crisis (SRC). Severe, uncontrolled hypertension and rapidly progressive renal failure characterize this syndrome. The onset of hypertension is abrupt and may be associated with retinal hemorrhages, retinal infarcts, and papilledema. Microangiopathic hemolytic anemia is common. The histologic picture in the kidney includes fibrinoid necrosis of the afferent arterioles (see Figure 1, p. 27), intimal proliferation, and/or collagen deposition in the interlobular and arcuate arteries of the kidney.1 Similar findings have been observed in normotensive scleroderma patients with normal renal function.
Before the 1980s, over 80% of SRC patients did not survive. The use of ACE inhibitors has drastically changed these figures. Nevertheless, the prognosis is still not good. Survival at one and five years is only 76% and 65%, respectively, for SRC patients treated with ACE inhibitors. Among SRC patients, 38% never require dialysis, 23% require temporary (2–18 months) dialysis, 19% require permanent dialysis, and 19% die within three months of diagnosis of SRC.2
Because of the relative lack of toxicity of ACE inhibitors and the severity of SRC, it seemed obvious that a trial of prophylaxis would be a good idea. However, recent data on patients with SRC suggest that those on ACE inhibitors at the onset of SRC may have worse outcomes than those not taking these drugs.3 One possible reason is that those on ACE inhibitors may have normotensive SRC and that diagnosis of the crisis may therefore be delayed in these patients. There was concern that a trial of ACE inhibitors may, indeed, carry some substantial risk for participating patients.
Before going ahead with such a trial, my colleagues and I thought that it would be useful to know what the outcome of SRC is in patients who are on ACE inhibitors at the time of onset of the crisis, versus those not on these drugs. However, to find enough SRC patients in any particular clinic—or even in a database—would be very difficult. The prevalence of scleroderma is only about 250 cases per million people and the incidence is only about 20 per million people per year. The latter statistic is important because renal crisis tends to occur only in relatively recent onset diffuse disease. Virginia Steen, MD, of Georgetown University Hospital in Washington, D.C., found that between 1972 and 1982, 60 (10%) of 596 progressive systemic sclerosis (SSc) patients developed SRC. The mean interval to SRC after the onset of scleroderma was 3.2 years in that retrospective study.3
Technology as a Tool for Safety Research
Our question was, Could the Internet help us perform this study? Many of us have probably already participated in studies using the Internet. Who has not received a set of questions on SurveyMonkey or a similar questionnaire program? John “Jack” Cush, MD, of Baylor Research Institute in Dallas, has performed several surveys using the ACR’s e-mail list, and he has used Internet technology to study various issues such as rheumatologists’ impressions of the use of musculoskeletal ultrasound. Many of us have also participated in Delphi exercises to obtain experts consensus opinions. However, my colleague Marie Hudson, MD, a rheumatologist at the Jewish General Hospital and assistant professor of Medicine at McGill University, both in Montreal, realized that the Internet could be used as a unique resource for the study of SRC, a rare complication of an uncommon disease.
Her goal was to assess whether the use of ACE inhibitors prior to the onset of SRC was associated with worse outcomes. Because SRC is so rare, she hypothesized that if she could enlist most of the world’s scleroderma experts to help her find and follow cases, she could find enough patients within a short time to answer the question.
The details of the methodology of this study have been published in the International Journal of Rheumatology.4
In September 2008, Dr. Hudson compiled a list of e-mail addresses for more than 550 rheumatologists with an interest in SSc from the Canadian Scleroderma Research Group, the Scleroderma Clinical Trials Consortium, the EULAR Scleroderma Trials and Research (EUSTAR) group, and other international collaborators, particularly from Colombia, Mexico, and Australia. They were contacted and invited to participate in the Web-based survey. Thereafter, every participating rheumatologist was sent an e-mail on every second Friday afternoon asking them simply, “Have you diagnosed a case of SRC in the past two weeks?” They were asked to check a yes or a no box. If the answer was no—and in most cases it was—that was all that was required of them for that period.
We calculated that a total sample of approximately 60 subjects would be needed … 15 months after the start of the survey, we had identified 76 incident cases of SRC.
If the answer was yes, they were then asked to answer a simple, short survey about their case; the survey required about five minutes to complete (see Figure 2, p. 24). The survey was developed and conducted using SurveyMonkey, a simple, inexpensive, Web-based survey tool. The survey allowed us to collect data on many important variables, such as patient demographics, disease characteristics, blood pressure and renal function prior to the onset of SRC, current use of ACE inhibitor or angiotensin receptor blockers (ARBs) at the time of diagnosis, concomitant medications, and signs and symptoms used to diagnose SRC.
The primary outcomes of interest were defined as death or dialysis at one year after the onset of SRC. One year after a patient is identified, a simple follow-up case report form is sent by e-mail to the recruiting rheumatologist. At study completion, the rates of dialysis or death at one year in SSc patients with SRC exposed to ACE inhibitors at the time that they developed SRC will be compared with the rates in those not on ACE inhibitors at the time they developed SRC. We calculated that a total sample of approximately 60 subjects would be needed to detect the estimated increased risk in poor outcomes in those exposed compared with those unexposed to ACE inhibitors.
Measure of Success
As of February 2010, 15 months after the start of the survey, we had identified 76 incident cases of SRC. Approximately half of the cases were from Canada and the United States and the other half from elsewhere around the world. Twenty-two percent of the patients were on an ACE inhibitor and 5% on an ARB at the onset of SRC. To date, we have collected one-year follow-up data on approximately one-third of the cohort. Of these, over 50% have died or remain on dialysis at one year. Collection of one-year follow-up data on the remainder of the patients is ongoing and when complete we will analyze the data for the outcomes of interest.
This extraordinary technology has permitted Dr. Hudson to collect enough cases in a very short time to study a rare event. In a recent review, Bost and colleagues discuss the use of Internet technology for drug discovery and development projects with the aim of identifying a cure for tropical infectious diseases.5 The authors point out that these “neglected diseases collaborations require a global, secure, multi-organization data-management solution, combined with a platform that facilitates communication and supports collaborative work.” Certainly in rheumatology we have seen a rapid growth in collaborations made possible through the use of Web-based resources. In Canada, the Canadian Scleroderma Research Group makes use of Web-based data entry to collect detailed yearly data on patients with scleroderma from over 15 sites. This has allowed us to collect information on over 1,100 patients in just over five years. Similar collaborations have permitted the collection of data from multiple sites on many of the rheumatic diseases and have fostered an explosion of research in relatively uncommon diseases.
Dr. Hudson’s use of Internet survey technology has been unique in that it has permitted the collection of data on a very rare condition in a short time. This study should serve notice that the rarity of a disease or complication should no longer be an impediment to its study. Research will no longer take place in one community or even in one country now that the Internet offers new possibilities for research.
Dr. Baron is chief of rheumatology at Jewish General Hospital in Montreal.
References
- Prisant LM, Loebl DH, Mulloy LL. Scleroderma renal crisis. J Clin Hypertens. 2003;5:168-170, 176.
- Steen VD, Medsger TA, Jr. Long-term outcomes of scleroderma renal crisis. Ann Intern Med. 2000;133: 600-603.
- Steen VD, Medsger TA Jr, Osial TA Jr, Ziegler GL, Shapiro AP, Rodnan GP. Factors predicting development of renal involvement in progressive systemic sclerosis. Am J Med. 1984; 76:779-786.
- Hudson M, Baron M, Lo E, Weinfeld J, Furst DE, Khanna D. An international, Web-based, prospective cohort study to determine whether the use of ACE inhibitors prior to the onset of scleroderma renal crisis is associated with worse outcomes-methodology and preliminary results. Int J Rheumatol. 2010;2010. pii: 347402.
- Bost F, Jacobs RT, Kowalczyk P. Informatics for neglected diseases collaborations. Curr Opin Drug Discov Devel. 2010;13:286-296.