Discussion
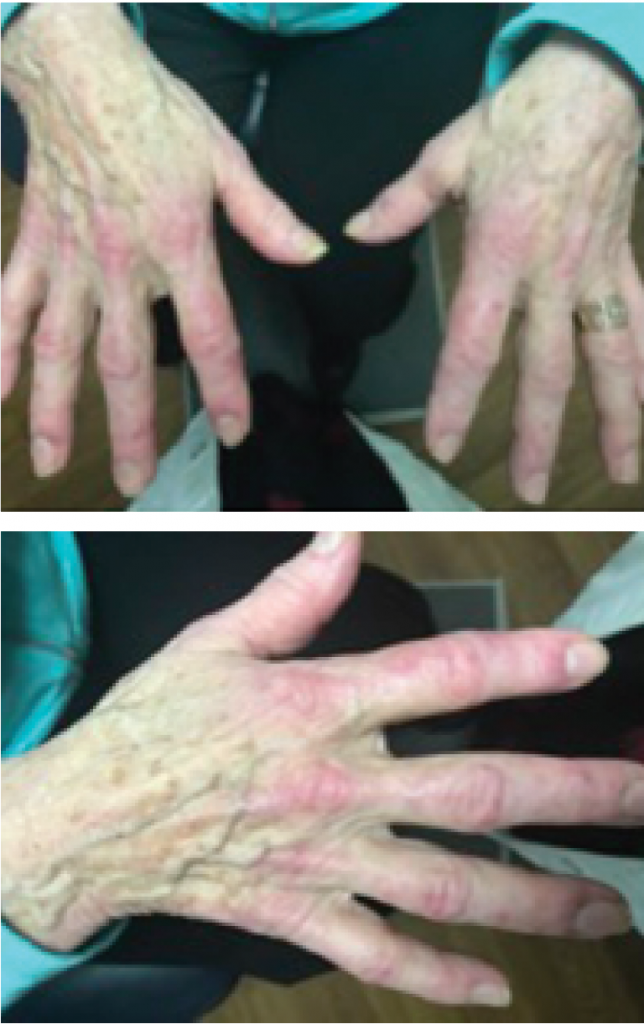
Figures 1 & 2: A hallmark skin manifestation of dermatomyositis includes Gottron’s papules, lesions consisting of erythematous to violaceous papules and plaques found over the metacarpophalangeal and interphalangeal joints.
Carcinoid syndrome is a paraneoplastic constellation of signs and symptoms mediated by the amplification of humoral factors by well-differentiated neuroendocrine tumors (NETs). These tumors, usually located in the digestive tract and lungs, secrete various polypeptides, biogenic amines and prostaglandins, such as serotonin and kallikrein. The annual incidence of NETs is anywhere from 2.5–5 per 100,000; the prevalence is estimated to be around 35 per 100,000. Postmortem exams have suggested asymptomatic NETs may be more common than reported, and reports of incidental discoveries of non-functioning tumors exsist. Symptoms associated with NETs include cutaneous flushing, secretory diarrhea, bronchospasms and cardiac valvular lesions. Typically, a NET is suspected when these symptoms are unexplained.1
Diagnosis of NETs involves laboratory testing, which includes serum chromogranin concentration, blood serotonin concentration, plasma 5-HIAA concentration and urinary excretion of serotonin and 5-HIAA. Various imaging modalities, such as computed tomography (CT) scan and magnetic resonance imaging, are used to isolate the NET. Indium-111 pentetreotide detects somatostatin receptors, which are expressed in high levels in NETs and are also useful to detect these tumors.
Traditionally, NETs have been classified by location, with hundreds of anatomic locations possible. To simplify classification, the World Health Organization has introduced a three-tier grading system, emphasizing tumor grade and not anatomic origin. NETs are staged depending on location, invasion and size.
Treatment and management for NETs depend on staging, grade and functionality of the tumor. Often, an important role for surgical intervention exsists to some degree. When possible, complete surgical excision of a NET is performed. After resection, patients should continue to be screened with intermittent serum and urine tests, as well as imaging.
Other treatments are aimed at palliation. For non-functioning, low-grade NETs, close observation can be offered. Symptomatic relief involves management with somatostatin analogs, such as octreotide. Anti-diarrheal medications may also be used. Cytotoxic chemotherapy is used for high-grade NETs. And radionuclide therapy has been shown to be effective for more aggressive NETs (i.e., if the tumor has high uptake of somatostatin). Radiofrequency ablation and cryoablation are also used to treat NETs. Gene therapies are currently in Phase 1 trials.2
NETs are very rarely linked to dermatomyositis. In literature, only a handful of case reports link NETSs to dermatomyositis, with the majority involving NETs in the liver or lungs. In most cases, treatment of the NET resolved the rheumatologic issue.
Dermatomyositis
Dermatomyositis is characterized by proximal, symmetric muscle weakness and skin eruption. Comprehensive epidemiologic data are lacking, because most studies are limited due to variations in age, gender and region. Moreover, most studies rely on physician billing and hospitalization databases, which may not capture all diagnoses.3 Nevertheless, Bendewald et al., using a 32-year, population-based retrospective study from Olmsted County, Minn., estimated that after age and sex adjustment, the incidence of dermatomyositis was about 1 per 100,000 people per year.4 The prevalence was around 20 cases per 100,000 people. Generally, dermatomyositis has a bimodal distribution that occurs in two peaks: one in the early teenage years and the second when the patient is 45–64 years old.
Five major criteria established by Bohan et al. in 1975 may be used to define dermatomyositis.5 These criteria include, 1) symmetrical weakness of the limb-girdle muscles and anterior neck flexors progressing from weeks to months with or without dysphagia or respiratory muscle involvemen; 2) muscle-biopsy evidence of necrosis of type I and II fibers, phagocytosis, regeneration with basophilia, large vesicular sarcolemmal nuclei and prominent nucleoli, atrophy in a peri-fascicular distribution, variation in fiber size, and an inflammatory exudate; 3) elevation in serum of skeletal-muscle enzymes, such as creatine phosphokinase and/or aldolase; 4) electromyographic triad of short, small, polyphasic motor units, fibrillations, positive sharp waves and insertional irritability, and bizarre, high-frequency repetitive discharges; and 5) dermatologic features, including heliotropic rash with periorbital edema and/or a scaly, erythematous dermatitis over the dorsum of the hands (e.g., Gottron’s papules) and involvement of knees, elbows, malleoli, face, neck or upper torso.
Three or four criteria, with the rash, can be considered definite for dermatomyositis. Two criteria with rash is considered probable, while one criterion plus rash can be considered possible.
It’s thought that 50–70% of dermatomyositis patients will have myositis-specific antibodies against nuclear or cytoplasmic antigens.6 Tumor-associated autoimmunity can also be directed against mutated forms of self-antigens or unrelated antigens associated with paraneoplastic syndromes. Examples include:


