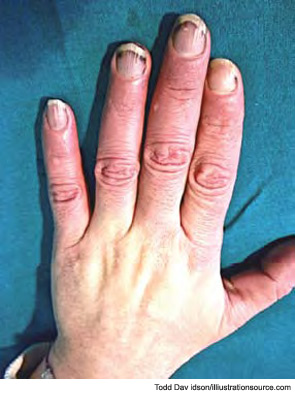
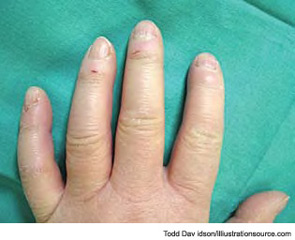

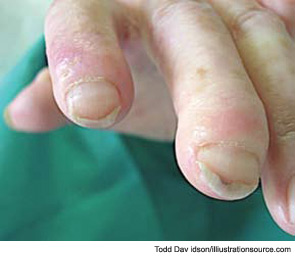
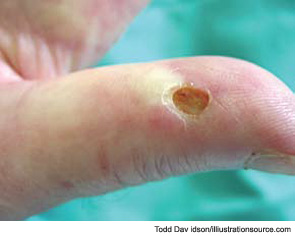
During a cold spell in the winter, a 58-year-old woman came into our rheumatology clinic complaining of discomfort from cold hands. She said that the problem started in 2004 when she was 53 years old; she gave an otherwise negative medical history (see Figure 1). She did not have symptoms related to any connective tissue disease, and her physical exam was unremarkable. There was no sclerodactyly, pitting scars, or dyspnea. Lung function analysis did not show signs of restrictive lung involvement (total lung capacity 99%, vital capacity 113%, diffusing lung capacity for carbon monoxide [DLCO] 109%). At echocardiography, there were no signs of abnormal pulmonary function (systolic pulmonary artery pressure was 25 mmHg). The laboratory showed low positivity for antinuclear antibodies (dilution 1:80), with immunofluorescence staining indicating a centromere pattern; immunoassay showed positivity for anticentromere antibodies.
Primary Raynaud’s Phenomenon or Systemic Sclerosis?
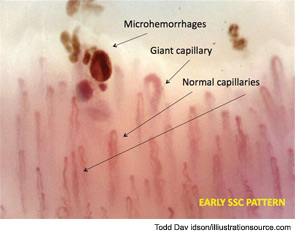
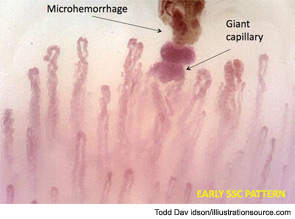
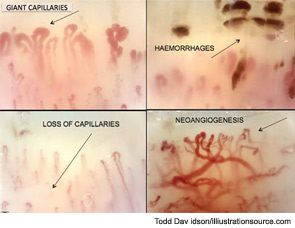
Further evaluation of the patient by nailfold videocapillaroscopic (NVC) analysis demonstrated the combination of giant capillaries and microhemorrhages, an “early” scleroderma pattern (see Figure 2). Since these findings are characteristic of microvessel damage in systemic sclerosis (SSc), the diagnosis turned to secondary Raynaud’s phenomenon.1 In this case, the diagnosis of early SSc was based on the criteria of LeRoy and Medsger, which were recently validated and include the scleroderma pattern by NVC and the presence of specific autoantibodies.2,3
This case is a clear example of early SSc (limited SSc); even in the absence of other non-Raynaud’s symptoms of disease, the diagnosis of SSc was possible because of the appearance of the classical morphological markers for microvessel damage (early NVC scleroderma pattern) that can characterize the beginning of the disease and have been validated.4
The Role of Microvascular Damage Assessment in SSc
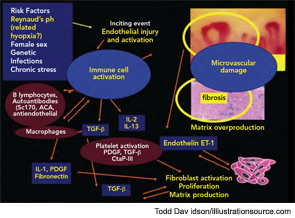
SSc is a rare progressive connective tissue disease characterized by vascular abnormalities/damage that is associated with diffuse fibrosis in the skin and progressive involvement of the internal organs. The origin of SSc appears multifactorial, although the patient’s gender has a strong impact. SSc is about eight times more common among women than men; the dramatic female predominance of the disease may reflect the role of estrogens in enhancing the immune responses.5 Most common in the third to fifth decades of life, SSc is rare in children. Although the basis for the age distribution is unknown, intriguing studies suggest a role of the aromatases in the periphery as a source of estrogen metabolites to enhance cell proliferation and fibrosis.6
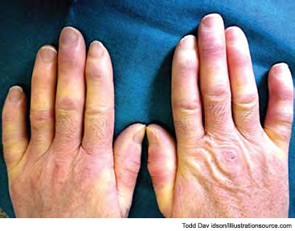
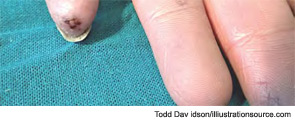
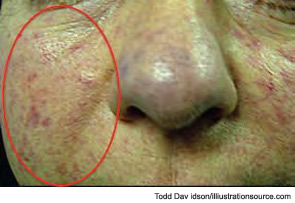
The most common initial symptoms and signs of SSc are vascular, such as Raynaud’s phenomenon, which is often long lasting, as well as insidious swelling of the distal extremities (e.g., puffy fingers). These features are followed by gradual thickening of the skin of the face and fingers along with skin ulcers (see Figures 3 and 4). The diagnosis of SSc in its earlier stage is clinical and includes the presence of Raynaud’s phenomenon together with capillaroscopic markers of microvasculature damage (e.g., giant capillaries and related microhemorrhages); biomarkers, namely SSc-specific serum autoantibodies, can help confirm the diagnosis.
Does Microvascular Change Reflect Pathophysiology?
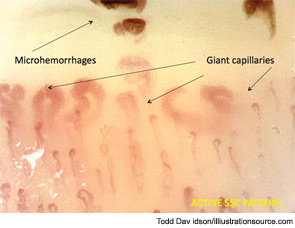
Peripheral microvascular damage in SSc is characterized by dynamic alterations in capillary structure that eventually can lead to a progressive decrease in capillary density. In the nailfolds, where the progression of the morphological changes can be detected by safe and noninvasive videocapillaroscopy (a microvideocamera, see Figure 5), capillary loops dilate homogenously (giant capillary) as they are damaged by the local immune/inflammatory reaction and gradually lose their red blood cell content (microhemorrhage); the loss of microvascular loops begins with their progressive collapse (see Figure 6). The reason that Raynaud’s phenomenon can produce an early NVC pattern relates to the effects of local ischemia from vasoconstriction; over time, this process may induce endothelial cell and tissue necrosis and damage (see Figure 7). The dynamic involvement of capillaries by the immunopathological process in SSc may be associated with worsening of the capillaroscopic pattern (from early to active pattern) that can be linked to overt disease symptoms (e.g., skin trophic lesions) (see Figure 8 and 9).
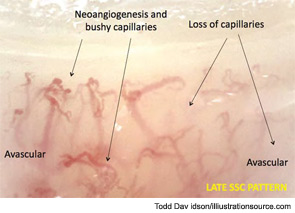
Thus, as the pathophysiological process of SSc progresses into fibrosis, capillaroscopic findings most likely reflect the effects of tissue hypoxia: massive capillary destruction, loss of capillaries, areas of avascularity, and bushy capillaries indicating neoangiogenesis (see Figure 10). This advanced stage of SSc characterizes the late capillaroscopic pattern. The classical features of neoangiogenesis also are evident on the skin of SSc patients with advanced disease (see Figure 11).
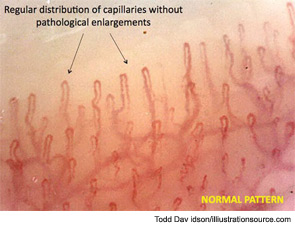
Although an early capillaroscopic pattern can occur in patients who have suffered from Raynaud’s phenomenon for many years, the term early is used because this pattern tends to occur initially; it also can be observed in patients with shorter disease duration than in those with active or late patterns. However, it must be recognized that capillaroscopic patterns are descriptive and that there is inevitable overlap between patterns. The differences between the pattern in SSc and the normal pattern of healthy subjects or patients with benign primary Raynaud’s phenomenon can be readily observed (see Figure 12).
The development of robust scoring systems to quantify the microvascular change is essential to the performance longitudinal studies. For this reason, a practical system to score these capillaroscopic alterations in patients with SSc has been recently proposed and validated.7,8 Interestingly, in studies on clinical findings over time, about 15%–20% of primary Raynaud’s phenomenon are considered to switch to secondary phenomenon over 12 years in terms of the development of evident clinical symptoms; conversely, when the clinical course is assessed with nailfold capillaroscopy, the transition from primary to secondary Raynaud’s (i.e., associated with SSc) can be detected in only 29 months.9,10
With the use of capillaroscopy for the differential diagnosis of Raynaud’s phenomenon and the follow-up of SSc patients, important studies have suggested links between microvasculature damage and laboratory markers of SSc. One of the first studies on the association between capillaroscopic change and circulating markers demonstrated a correlation between serum levels of the soluble adhesion molecule E-selectin and capillary loss, especially in patients with early disease (within 48 months of diagnosis); this finding suggests that serum E-selectin levels could be a biomarker of disease activity in SSc.11 Serum levels of the tissue kallikrein, a mediator that can act as an angiogenic agent in the microcirculation, also were found to be higher in SSc patients with early and active capillaroscopic patterns compared with those with the late pattern.12 Interestingly, the highest plasma levels of endothelin-1 (ET-1) can be detected in the more advanced stage of the SSc microangiopathy, namely, the late NVC pattern; this pattern is characterized by capillary loss and increased tissue fibrosis. Together, these results may support the involvement of ET-1 in the progression of disease from microvascular to fibrotic SSc damage.13 In this regard, ET-1 could mediate SSc pathogenesis by promoting vascular endothelial cell proliferation, vasoconstriction, smooth muscle hypertrophy, irreversible vascular remodeling in the lungs, and by increasing fibroblast synthesis of type I and type III collagen and fibronectin by a receptor-dependent mechanism.14
Capillaroscopic Analysis to Predict Clinical Complications
Recently, several investigations have evaluated the association of nailfold capillaroscopic patterns with both demographic and clinical features in SSc patients. Data from these studies have shown that SSc microangiopathy correlates with disease subset and disease severity, primarily of peripheral vascular, skin, and lung involvement. In particular, studies indicate that SSc patients with the late NVC pattern have an increased risk of active disease and the occurrence of moderate to severe skin or visceral involvement compared with patients with early and active capillaroscopic patterns.15
Skin ulcers are a common vascular complication of progressive SSc and can occur in association with the late NVC pattern; this pattern is characterized by the presence of avascular areas, indicative or consistent with tissue necrosis (see Figure 13). An association between trophic lesions of the skin and capillary loss, as assessed by semiquantitative scoring, has also been reported.16 In fact, loss of capillaries may be important in tissue hypoxia and, in patients with recent-onset Raynaud’s phenomenon, the appearance of rapidly progressive capillary loss by capillaroscopy may represent the first evidence of severe SSc with destruction of microvessels.17
Implications of Progressive Capillary Loss for Complications in SSc
To evaluate the role of the capillaroscopic analysis for predicting the development of skin ulcers of SSc patients, several indices have been proposed, particularly for progressive capillary loss (see Figure 14). A recent study presented a risk index that might predict new digital ulcers by NVC analysis in patients with SSc; however, this index may be too complex for routine use and has not yet been validated.18 Subsequently, another study has found that capillary dimensions and loss of capillaries are strongly associated with digital ulceration; a significant correlation with increased serum ET-1 level also was confirmed in patients with SSc.19 Recently, using a reliable and simple NVC parameter that can be scored (semi-)quantitatively (mean score of capillary loss), a simple prognostic index for use in SSc clinics was obtained in consecutive patients for prediction of digital trophic lesions.20
Regarding the predictive value of capillaroscopy in visceral involvement, a seminal study showed that reduced nailfold capillary density in SSc patients with established pulmonary arterial hypertension (PAH) might have pathogenic significance and allow detection of the subset of patients with this severe disease manifestation (almost 20%) at an early stage in its progression.21 A more recent study showed that the association between ground-glass lung opacities and higher capillaroscopic avascular scores (loss of capillaries) is particularly strong in SSc patients with a disease duration of less than five years.22
The significance of capillary nailfold changes (i.e., loss of capillaries) in SSc patients with and without PAH was addressed in a larger study that included not only patients with SSc but also patients with idiopathic PAH.23 In this study, the capillary number was significantly lower in patients with SSc and PAH compared with patients with SSc but without PAH; loop dimensions, however, were similar. This finding suggests that loop dimensions may be neither a reliable nor informative parameter for prognostic purposes. On the other hand, the data in this study indicated the number of capillaries in healthy control subjects is significantly higher than those in patients with SSc without PAH, SSc patients with PAH, and patients with idiopathic PAH. Interestingly, capillary density was negatively and significantly correlated with mean pulmonary arterial pressure at rest in patients with SSc and PAH and in patients with idiopathic PAH.23 Thus, a reduction in nailfold capillary density (late SSc pattern) appears to correlate with the severity of PAH in both SSc and idiopathic PAH.
Effects of Therapy on Microvascular Damage and Videocapillaroscopy Findings
Current treatments for SSc have very limited efficacy. Nevertheless, the management of Raynaud’s phenomenon may slow the progression, including the remodelling of digital microvascular abnormalities that can impair perfusion and cause tissue hypoxia due to the loss of capillaries.
Capillaroscopy has an important role in this setting since several currently available drugs can affect vascular remodeling. Recently, we obtained exciting results (currently being prepared for publication) that show a significant reduction of the capillary loss, as evaluated by videocapillaroscopy in SSc patients treated with a combination of intravenous prostanoids and endothelin receptor blockers. Other provocative data on this issue relate to the reduction of the microvasculature damage (regression of the early capillaroscopic markers [i.e., giant capillaries and microhemorrhages] and reversal of the qualitative patterns) observed with immunosuppressive therapies (see Figure 15). Since immunosuppressive therapies which block immune activity may prevent secondary tissue injury and fibrosis, cyclosporin has been tested as a treatment in SSc patients. Studies on the effect of this agent have shown a substantial impact on clinical symptoms after 12 months of treatment with, interestingly, a concomitant reversal of the capillaroscopic advanced patterns.24,25 Similarly, cyclophosphamide administration was found to be significantly associated with a regression of the capillaroscopic patterns, and none of the SSc patients who received the drug demonstrated worsening of microvascular lesions.26 Of note in this study, the progression of the capillaroscopic pattern was inversely and significantly correlated with cyclophosphamide treatment.
In a further study, intensive immunosuppressive treatment with cyclophosphamide was shown to ameliorate microvascular damage in patients with SSc as observed by rapid improvement of the NVC pattern.27 Together, these observations indicate a role of NVC in assessing treatment responses in SSc.
Further Perspectives and Conclusions
In 2001, a study reported that the sensitivity of the ACR criteria in identifying patients with limited SSc improved from 34% to 89% with the addition of nailfold capillary abnormalities and the presence of visible telangiectasias.9 More recently, in another study, the sensitivity of the criteria increased from 67% to 99% with the addition of nailfold capillary abnormalities identified using a dermatoscope and visible telangiectasias.6 At present, an updating of the classification criteria for SSc including the capillaroscopic analysis is in progress. In an effort to extend and refine the seminal studies on capillaroscopy performed in the United States 35 years ago, an ACR study group was formed in 2010.28 In addition, a practical atlas on capillaroscopy in rheumatic diseases is now available, signifying the interest in this technique and a guide for lesion assessment.29
In conclusion, a final message for readers of this review is to consider capillaroscopic analysis as a fundamental element to achieve at least two goals in the evaluation of patients presenting with symptoms of cold hands: 1) differential diagnosis between primary and secondary Raynaud’s phenomenon; and 2) early diagnosis of SSc. Further, capillaroscopic analysis can provide valuable information to help assess disease prognosis and evaluate the response to therapy in patients with SSc. From its beginning, capillaroscopy has represented a unique imaging tool that is simple, objective, totally safe (it is a microscope) and can help rheumatologists both document and understand the complex vascular phenomena. As recent study results show, the promise of capillaroscopy remains great, with its continued use to study patients with SSc hopefully providing new insights into one of the most serious and confusing diseases in all of medicine. Capillaroscopy also may facilitate much-needed advances in treatment.
Dr. Cutolo is professor of rheumatology, director of the Research Laboratories and Academic Unit of Clinical Rheumatology, and director of the Postgraduate Academic School of Rheumatology at the University of Genova in Italy; past chair of the EULAR Standing Committee for Education and Training (ESCET); and president-elect of EULAR.
References
- Cutolo M, Sulli A, Smith V. Assessing microvascular changes in systemic sclerosis diagnosis and management. Nat Rev Rheumatol. 2010;6:578-587.
- LeRoy EC, Medsger TA Jr. Criteria for the classification of early systemic sclerosis. J Rheumatol. 2001;28:1573-1576.
- Koenig, M, Joyal F, Fritzler MJ, et al. Autoantibodies and microvascular damage are independent predictive factors for the progression of Raynaud’s phenomenon to systemic sclerosis: A twenty-year prospective study of 586 patients, with validation of proposed criteria for early systemic sclerosis. Arthritis Rheum. 2008;58:3902-3912.
- Cutolo M, Capellino S, Straub RH. Oestrogens in rheumatic diseases: Friend or foe? Rheumatology (Oxford). 2008;47:3:iii2-5.
- Herrick AL, Cutolo M. Clinical implications from capillaroscopic analysis in patients with Raynaud’s phenomenon and systemic sclerosis. Arthritis Rheum. 2010;62:2595-2604.
- Cutolo M, Straub RH, Bijlsma JW. Neuroendocrine-immune interactions in synovitis. Nat Clin Pract Rheumatol. 2007;3:627-634.
- Sulli A, Secchi ME, Pizzorni C, et al. Scoring the nailfold microvascular changes during the capillaroscopic analysis in systemic sclerosis patients. Ann Rheum Dis. 2008;67:885-887.
- Smith V, Pizzorni C, De Keyser F, et al. Reliability of the qualitative and semiquantitative nailfold videocapillaroscopy assessment in a systemic sclerosis cohort: A two-centre study. Ann Rheum Dis. 2010;69:1092-1096.
- Hirschl M, Hirschl K, Lenz M, et al. Transition from primary Raynaud’s phenomenon to secondary Raynaud’s phenomenon identified by diagnosis of an associated disease: Results of ten years of prospective surveillance. Arthritis Rheum. 2006;54:1974-1981.
- Cutolo M, Pizzorni C, Sulli A. Identification of transition from primary Raynaud’s phenomenon to secondary Raynaud’s phenomenon by nailfold videocapillaroscopy: Comment on the article by Hirschl et al. Arthritis Rheum. 2007;56:2102-2103.
- Valim V, Assis LS, Simoes MF, et al. Correlation between serum E-selectin levels and panoramic nailfold capillaroscopy in systemic sclerosis. Braz J Med Biol Res. 2004;37:1423-1427.
- Del Rosso A, Distler O, Milia AF, et al. Increased circulating levels of tissue kallikrein in systemic sclerosis correlate with microvascular involvement. Ann Rheum Dis. 2005;64:382-387.
- Sulli A, Soldano S, Pizzorni C, et al. Raynaud’s phenomenon and plasma endothelin: Correlations with capillaroscopic patterns in systemic sclerosis. J Rheumatol. 2009;36:1235-1239.
- Soldano S, Montagna P, Villaggio B, et al. Endothelin and sex hormones modulate the fibronectin synthesis by cultured human skin scleroderma fibroblasts. Ann Rheum Dis. 2009;68:599-602.
- Caramaschi P, Canestrini S, Martinelli N, et al. Scleroderma patients nailfold videocapillaroscopic patterns are associated with disease subset and disease severity. Rheumatology (Oxford). 2007;46:1566-1569.
- Smith V, Pizzorni C, De Keyser F, et al. Validation of the qualitative and semiquantitative assessment of the scleroderma spectrum patterns by nailfold videocapillaroscopy: Preliminary results [abstract]. Arthritis Rheum. 2009;60 Suppl:S164-S165.
- Cutolo M, Ferrone C, Pizzorni C. et al. peripheral blood perfusion correlates with microvascular abnormalities in systemic sclerosis: A laser-doppler and nailfold videocapillaroscopy study. J Rheumatol. 2010;37:1174-1180.
- Sebastiani M, Manfredi A, Colaci M, et al. Capillaroscopic skin ulcer risk index: A new prognostic tool for digital skin ulcer development in systemic sclerosis patients. Arthritis Rheum. 2009;61:688-694.
- Kim HS, Park MK, Kim HY, et al. Capillary dimension measured by computer-based digitalized image correlated with plasma endothelin-1 levels in patients with systemic sclerosis. Clin Rheumatol. 2010;29:247-254.
- Smith V, De Keyser F, Pizzorni C, et al. Nailfold capillaroscopy for day-to-day clinical use: Construction of a simple scoring modality as a clinical prognostic index for digital trophic lesions. Ann Rheum Dis. 2011;70:180-183.
- Ong YY, Nikoloutsopoulos T, Bond CP, et al. Decreased nailfold capillary density in limited scleroderma with pulmonary hypertension. Asian Pac J Allergy Immunol. 1998;16:81-86.
- Bredemeier M, Xavier RM, Capobianco KG, et al. Nailfold capillary microscopy can suggest pulmonary disease activity in systemic sclerosis. J Rheumatol. 2004;31:286-294.
- Hofstee HM, Noordegraaf AV, Voskuyl AE, et al. Nailfold capillary density is associated with the presence and severity of pulmonary arterial hypertension in systemic sclerosis. Ann Rheum Dis. 2009;68:191-195.
- Filaci G, Cutolo M, Scudeletti M, et al. Cyclosporin A and iloprost treatment of systemic sclerosis: Clinical results and interleukin-6 serum changes after 12 months of therapy. Rheumatology (Oxford). 1999;38:992-996.
- Filaci G, Cutolo M, Basso M, et al. Long-term treatment of patients affected by systemic sclerosis with cyclosporin A. Rheumatology (Oxford). 2001;40:1431-1434.
- Caramaschi P, Volpe A, Pieropan S, et al. Cyclophosphamide treatment improves microvessel damage in systemic sclerosis. Clin Rheumatol. 2009;28:391-395.
- Aschwanden M, Daikeler T, Jaeger KA, et al. Rapid improvement of nailfold capillaroscopy after intense immunosuppression far systemic sclerosis and mixed connective tissue disease. Ann Rheum Dis. 2008;67:1057-1059.
- Maricq HR, Spencer-Green G, LeRoy EC. Skin capillary abnormalities as indicators of organ involvement in scleroderma (systemic sclerosis), Raynaud’s syndrome and dermatomyositis. Am J Med. 1976;61:862-870.
- Cutolo M, Smith V, Sulli A, eds. Atlas of capillaroscopy in rheumatic diseases. Elsevier (Milan,Italy), 2010.

