Ironically, chronic exposure to minocycline has also been associated with a variety of autoimmune syndromes, including drug-induced lupus, autoimmune hepatitis, serum sickness and vasculitis.1 Minocycline is associated with an 8.5-fold increased risk of drug-induced lupus.2 Minocycline and nitrofurantoin are implicated in 90% of cases of drug-induced autoimmune hepatitis.3 Minocycline-induced vasculitis is much less common and, therefore, may not receive appropriate consideration.
We present a case of polyarteritis nodosa (PAN), primarily affecting the lower extremity peripheral nerves and skin, that improved only after minocycline was recognized as the culprit and stopped.
Case Presentation
A 40-year-old man presented with 10 days of pain, stiffness and swelling in his hands, wrists, shoulders, ankles and feet, along with generalized myalgias. He had a history of acne vulgaris, which had been treated with minocycline for the previous three years. His past medical history was otherwise unremarkable.
He denied having a rash, fever, chills, dyspnea, abdominal pain, chest pain or paresthesias. He had tender, non-swollen joints in his fingers, wrists, shoulders, ankles and feet.
His initial laboratory tests showed an erythrocyte sedimentation rate of 17 mm/hr (reference range: 0–15 mm/hour in men younger than 50) and a C-reactive protein level of 3.8 mg/dL (reference range: <0.5 mg/L); rheumatoid factor (RF) and anti-nuclear antibodies (ANA) were absent. He was started on 20 mg/day of prednisone, which was tapered over four weeks and quickly felt better. He was referred to our rheumatology service for further evaluation.
At the time of his initial consult visit with us, he had finished his prednisone taper and was experiencing recurrence of his joint pain. He had no rash or joint swelling on examination. Laboratory tests were remarkable for C-reactive protein of 2.3 mg/dL, a positive ANA (titer 1:160, speckled pattern) and an elevated white blood count of 12.3×103/mcL (see Table 1). He did not have antibodies against extractable nuclear antigens, anti-nuclear cytoplasmic antibodies (ANCA), RF or anti-cyclic citrullinated peptide antibodies (ACPA). His muscle enzymes and urinalysis were both normal, as was his chest X-ray.
Patients with minocycline-associated vasculitic neuropathy respond quickly (within four to 12 weeks) to the antibiotic’s withdrawal.
He was placed back on prednisone 40 mg/day with a slow taper. Hydroxychloroquine 400 mg/day was started. Within eight weeks, he was able to reduce his prednisone dose to 5 mg/day without recurrence of his joint symptoms; however, he developed shooting pain, numbness and tingling in the feet and ankles. He also noticed an episodic, fleeting, erythematous exanthema on his lower extremities.
Table 1: Laboratory Tests at Presentation to the Rheumatologist
| Laboratory | Test Result* | Reference Range |
|---|---|---|
| White cell count | 12.3x103/mcL | 4.0x103/mcL–10.0x103/mcL |
| Hemoglobin | 14.8 gm/dL | 13.5–17.5 gm/dL |
| Platelet | 312x103/mcL | 140x103/mcL–450x103/mcL |
| Serum creatinine | 0.84 mg/dL | 0.64–1.27 mg/dL |
| Aspartate amniotransferase (AST) | 27 IU/L | 15–41 IU/L |
| Alanine aminotransferase (ALT) | 28 IU/L | 50–280 IU/L |
| Uric acid | 3.3 mg/dL | 1.2–7.6 mg/dL |
| Creatine phosphokinase (CK) | 81 IU/L | 50–280 IU/L |
| Alsolase | 4.1 units/L | 1.2–7.6 units/L |
| C-reactive protein | 2.3 mg/L | <0.5 mg/L |
| Anti-nuclear antibody (ANA) | 1:160 titer (speckled) | <1:80 titer |
| Anti-SSA (Ro) | 6 units | <20 units |
| Anti-SSB (La) | 2 units | <20 units |
| Anti-double-stranded DNA (Crithidea indirect fluorescent antibody) | negative | negative |
| Anti-Smith | 4 units | <20 units |
| Anti-RNP | 16 units | <20 units |
| C3 complement | 129 mg/dL | 79–152 mg/dL |
| C4 complement | 26 mg/dL | 15–46 mg/dL |
| Anti-neutrophil cytoplasmic antibody (ANCA) | <1:20 titer | <1:20 titer |
| Anti-cyclic citrullinated peptide IgG (ACPA) | 6 units | <20 units |
| Rheumatoid factor | <15 IU/mL | <15 IU/mL |
| Hepatitis B surface antigen | negative | negative |
| Hepatitis C antibody | nonreactive | nonreactive |
| Lyme antibody | 0.09 | <0.9 |
| Urine blood | <1/HPF | 0–2/HPF |
| Urine protein | negative | negative |
| *abnormal findings indicated in bold |
His prednisone was increased back to 40 mg/day, and electromyogram (EMG)/nerve conduction studies were ordered. Further laboratory testing demonstrated normal serum protein electrophoresis, hemoglobin A1c, B12 and thyroid-stimulating hormone. He did not have antibodies against syphilis, hepatitis B or hepatitis C. His EMG/nerve conduction test showed bilateral sensorimotor polyneuropathy with absent sural and diminished posterior tibial nerve responses, consistent with mononeuritis multiplex.
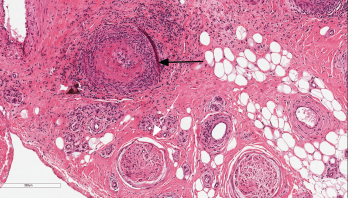
Figure 1: Nerve Biopsy
This image depicts the sural nerve biopsy with hematoxylin and eosin staining at 100x magnification. The black arrow indicates necrotizing vasculitis of epineural arterioles and muscular arteries with fibrinoid necrosis. The infiltrate is a mixture of neutrophils and mononuclear cells. There is luminal thrombosis with recannulation. Nerves (white arrow) show mildly thickened perineurium, but are relatively unaffected by inflammation.
Sural nerve and gastrocnemius biopsies were performed. Microscopic pathology showed a perineural, large arteriolar, necrotizing vasculitis consistent with PAN (see Figure 1).
We diagnosed vasculitic neuropathy and started our patient on monthly intravenous cyclophosphamide (500 mg/m2). After three months of treatment, he was not able to reduce his prednisone dose below 30 mg/day without worsening, painful dysesthesias in his feet and lower legs; additionally, his C-reactive protein remained elevated (5.8–8.9 mg/dL).
Another three months of intravenous cyclophosphamide at 750 mg/m2 was administered without improvement in his symptoms, C-reactive protein levels or prednisone requirement. Azathioprine was then tried, without any clinical benefit.
The previously fleeting leg rash became more persistent, and was joined by erythematous, reticulated macules on his arms and legs, and scattered purpuric papules. He also developed livedo racemosa, as well as slate-gray discoloration of the anterior lower legs, likely due to minocycline hyperpigmentation. A skin biopsy of an erythematous papule on his posterior left thigh demonstrated normal epidermis and superficial dermis with deep dermis and panniculus featuring intramural mixed inflammatory infiltrates of lymphocytes, histiocytes and neutrophils in medium and large vessels (see Figure 2). Direct immunofluorescence was negative for IgG, IgA, IgM and C3. The pathologic description was read as consistent with polyarteritis nodosa (PAN).
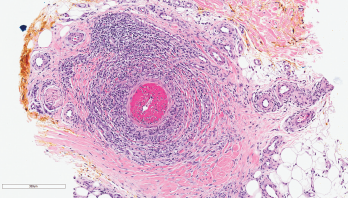
Figure 2: Skin Biopsy
This image depicts the left posterior thigh biopsy of the soft tissue with hematoxylin and eosin staining at 100x magnification and shows necrotizing vasculitis of a deep dermal muscular artery with fibrinoid necrosis. The infiltrate is predominantly neutrophilic, and there is marked thrombosis of the arterial lumen. Concurrent direct immunofluorescence studies were negative.
Minocycline was identified as a potential trigger for the patient’s vasculitis and was discontinued. Within 30 days after stopping the minocycline, his rash resolved, his pain significantly improved and his C-reactive protein normalized (0.3 mg/dL). He received no more cyclophosphamide. Azathioprine was stopped, and prednisone was successfully weaned to 5 mg/day within one month and was stopped entirely by six months. Although he experienced persistent dysesthesias in his feet, presumed to be from permanent nerve damage, all of his other symptoms stabilized. At his three-year follow-up, he had experienced no further rashes or arthralgias, and his C-reactive protein remained normal.
Discussion
Minocycline is a synthetic, tetracycline-derived, broad-spectrum antibiotic that has been used for more than 30 years. It is active against a wide range of aerobic and anaerobic gram-positive and gram-negative bacteria, and against other microorganisms, including Rickettsia, Chlamydia, Plasmodium and Mycoplasma pneumoniae. Minocycline also has anti-inflammatory properties that have been studied, with variable success, for the treatment of rosacea, bullous and neutrophilic dermatoses, pyoderma gangrenosum, sarcoidosis, aortic aneurysms, cancer metastasis, periodontitis and autoimmune disorders, such as rheumatoid arthritis and scleroderma.4
Proposed mechanisms of immune modulation include inhibition of enzymes (e.g., metalloproteinases and nitrous oxide synthetase); inhibition of neutrophil apoptosis; immune cell activation, proliferation and chemotaxis; and abnormal production of reactive metabolites.4
Our case & those reviewed underscore the importance of recognizing the association of positive ANA & P-ANCA tests with long-term minocycline use.
Rarely, minocycline has also been reported to trigger various autoimmune diseases. Drug-induced lupus stemming from minocycline use has been well documented in dermatology and rheumatology literature since the first case reports in 1992, almost 20 years after the drug became available on the market.5 Clinical manifestations develop, on average, around two years after initial exposure to the drug and are usually limited to dermatologic and musculoskeletal systems.6 All patients have positive ANA tests, but anti-histone antibodies are uncommon. Minocycline is the only antibiotic in the tetracycline class associated with drug-induced lupus.
An association between minocycline and autoimmune hepatitis is also well recognized. Liver injury typically occurs within two years after starting the drug.1 Laboratory abnormalities typically include high-titer ANA, peripheral ANCA (P-ANCA) and polyclonal gammopathies. Disease manifestations resolve shortly after minocycline discontinuation. Minocycline has also been associated with eosinophilic pneumonitis, serum-sickness and Sweet’s syndrome.1
Vasculitic neuropathies are well described in the literature and have numerous secondary causes, mainly related to other diseases.7 Medication-associated vasculitic neuropathy is not well represented in the literature. A case-based review in 2013 described 15 patients with minocycline-induced vasculitis.8 Ten patients had ANA, eight patients had P-ANCA, and four had MPO-ANCA. Most patients had skin involvement, with livedo reticularis and/or subcutaneous nodules. Seven patients had musculoskeletal symptoms (arthralgias and myalgias). One patient had testicular involvement. One patient had a sural nerve biopsy that showed vasculitis with thrombosis in a medium-sized artery.
In 2012, a group from the Mayo Clinic described nine patients with minocycline-associated polyarteritis nodosa-like vasculitis and reviewed 12 other cases in the literature.9 Patient ages in the Mayo series ranged from 18–55 years.
Four patients in the Mayo series had skin involvement, and four patients had other organs that were pathologically shown to be sites of active vasculitis, including gallbladder (one patient), renal artery (one patient) and testicle (one patient). Two patients had mononeuritis multiplex (one associated with the gallbladder and one with isolated peripheral nerve disease). Constitutional symptoms (fever, malaise, arthralgias, myalgias) were present in eight patients. Three patients were ANA positive and nine patients were P-ANCA positive (MPO-ANCA in two and PR3-ANCA in one).
Patients were excluded from the series if they were found to meet criteria for ANCA-associated vasculitis or ANA-associated connective tissue diseases. The median duration of minocycline use in this series was two years (range: one to four years). Three patients were treated only by minocycline withdrawal. Six patients required additional treatment with immunosuppressants (e.g., prednisone, cyclophosphamide, mycophenolate mofetil, azathioprine, sulfasalazine and dapsone).
In the other 12 cases, the patients’ ages ranged from 18–70 years. Ten had skin involvement, one had testicular pain (presumed to be from vasculitis), and one had isolated mononeuritis multiplex. Eight patients were ANCA positive (six were P-ANCA positive, of whom two were MPO positive), four had borderline ANCA “type unknown.” The median duration of minocycline use was 2.5 years, and all cases were reported as resolved by stopping the minocycline therapy.
We identified and reviewed 11 additional cases of vasculitic neuropathy presenting with primary neurologic involvement (see Table 2, p. 30).10–17 Ages ranged from 17–70 years. Six cases involved women, and five involved men. Eight patients presented with mononeuritis multiplex (seven cases involved lower extremities and one involved an upper extremity). Three patients presented with midbrain strokes (one with concomitant mononeuritis multiplex). One patient presented with transient hemiparesis and was found to have vertebral branch arteritis. Only one patient had skin involvement. Six patients experienced constitutional symptoms, including fevers, chills, fatigue and arthralgias. The average time of exposure to minocycline before symptoms developed was a little less than two years. Eight patients were ANA positive (one was anti-Ro positive, and one had anti-histone antibody). Four were P-ANCA positive (of whom, two were MPO positive). Eight patients were treated with immunosuppressant medications (including corticosteroids, azathioprine, methotrexate and cyclophosphamide). Resolution of active disease occurred in all patients after minocycline was discontinued, with an average response time of 10 weeks.
Table 2: Summary of Cases of Minocycline-Associated Isolated Vasculitic Neuropathy & Response Time After Discontinuing Minocycline
| Patient | Ref. | Age/Sex | Symptoms | Minocycline Use | ANA | ANCA | Diagnosis | Additional Treatment | Response Time |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 10 | 28/F | Lower extremity weakness | 2 weeks | pos (anti-RO) | neg | Sural nerve biopsy | CS | 3 months |
| 2 | 11 | 17/M | Diplopia from 3rd nerve palsy | ? | neg | P-ANCA (MPO pos) | MRI (left midbrain ischemia) | CS | 6 weeks |
| 3 | 9 | 40/F | Fever, chills, fatigue, arthralgias, mononeuritis multiplex, vision loss, diplopia | >1 year | pos (ENA neg) | P-ANCA (MPO/PR3 neg) | Sural nerve biopsy | CS, CYC, AZA | ? |
| 4 | 12 | 27/M | Right-sided numbness, urine retention, gait imbalance, leg fatiguability | 1 year | neg | P-ANCA (MPO pos) | Cervical nerve biopsy | CS | 6 months |
| 5 | 13 | 70/F | Fever, arthralgias, weight loss, melena, mononeuritis multiplex | 8 months | pos (ENA neg) | neg | Sural nerve biopsy | ? | ? |
| 6 | 14 | 47/M | Fever, myalgias, arthralgias, LE paresthesias, testicular pain | 3 years | pos (ENA neg) | neg | EMG (bilateral sural neuropathy) | None | 4 weeks |
| 7 | 15 | 26/F | Pontine stroke, LE nodules, livedo, arthralgias | 3 years | pos (histone Ab) | P-ANCA (MPO/PR3 neg) | Skin biopsy | None | 3 weeks |
| 8 | 16 | 17/F | UE mononeuritis multiplex, medullary stroke | 3 years | pos | ? | Radial nerve biopsy | CS | 2 months |
| 9 | 16 | 33/M | Left foot pain/paresthesias, myalgias, arthalgias, fatigue, fever | 2 years | pos | ? | Gastroc muscle biopsy | CS, CYC | 3 months |
| 10 | 16 | 28/F | Mononeuritis multiplex | 2 weeks | pos (anti-RO) | ? | Sural nerve biopsy | CS | 3 months |
| 11 | 17 | 17/M | Mononeuritis multiplex, arthralgias | 18 months | pos (ENA neg) | neg | Sural nerve biopsy | CS, MTX | 2 months |
Key: ANCA: anti-neutrophil cytoplasmic antibody; P-ANCA: perinuclear ANCA; ANA: anti-nuclear antibody; ENA: extactable nuclear antigen; MPO: myeloperoxidase; PR3: proteinase 3;
In a 2007 dermatology cross-sectional study, ANCA was found in 7% of minocycline-exposed acne patients compared with none in the unexposed cohort group.18 In this study, no statistical difference in the prevalence of ANA positivity between patients exposed (13%) or not exposed (11%) to minocycline was found. However, higher titers of ANA (1:160 or higher) were found in the minocycline-exposed group (45%) than in the unexposed group (12%).
Conclusion
We present an unusual case of a 40-year-old who had been taking minocycline for three years to treat acne vulgaris. He developed acute, bilateral, lower extremity mononeuritis multiplex-associated vasculitis meeting the ACR criteria for PAN.19 As with minocycline-
associated systemic lupus and autoimmune hepatitis and other drug-induced autoimmune diseases, a high index of suspicion and prompt removal of the offending agent is key to clinical remission.
Similarities between our case and cases of minocycline-associated primary vasculitic neuropathy found in the literature may assist with this recognition (see Table 3). Almost all cases involved the long-term (two years on average) use of minocycline for acne vulgaris. Skin manifestations, including livedo reticularis and purpuric papules, were present in our patient and common in the cases previously reported, as were symptoms of generalized arthralgias and myalgias.
Table 3: Common Features of Minocycline-Associated Vasculitis
| • Several years (average 2) of patient exposure to minocycline before symptoms appear; |
| • Skin involvement (livedo reticularis and cutaneous nodules); |
| • Myalgias and arthralgias; |
| • P-ANCA positivity without ANCA vasculitis features; and |
| • Rapid clinical improvement after discontinuing minocycline. |
Our case and those reviewed underscore the importance of recognizing the association of positive ANA and P-ANCA tests with long-term minocycline use. Long-term immunosuppressive treatment is not necessary for these patients. All patients reported in the literature responded quickly (within four to 12 weeks) to withdrawal of minocycline.
Martin Garber, DO, is a lecturer in the Division of Rheumatology, Department of Internal Medicine, University of Michigan, Ann Arbor. He is also an instructor in internal medicine in the Division of Rheumatology at the University of Michigan Medical School.
David Fivenson, MD, is the director of Fivenson Dermatology, Ann Arbor, Mich.
References
- Elkayam O, Yaron M, Capsi D. Minocycline-induced autoimmune syndromes: An overview. Semin Arthritis Rheum. 1999 Jun;28(6):392–397.
- Sturkenboom MC, Meier CR, Jick H, Stricker BH. Minocycline and lupuslike syndrome in acne patients. Arch Intern Med. 1999 Mar 8;159(5):493–497.
- Czaja AJ. Drug-induced autoimmune-like hepatitis. Dig Dis Sci. 2011 Apr;56(4):958–976.
- Garrido-Mesa N, Zarzuelo A, Galvez J. Minocycline: Far beyond an antibiotic. Br J Pharmacol. 2013 May;169(2):337–352.
- Matsuura T, Shimizu Y, Fujimoto H, et al. Minocycline-related lupus. Lancet. 1992 Dec 19–26;340 (8834–8835):1553.
- Schlienger RG, Bircher AJ, Meier CR. Minocycline-induced lupus. A systemic review. Dermatology. 2000;200(3):223–231.
- Gwathmey KG, Burns TM, Collins MP, Dyck PJB. Vasculitic neuropathies. Lancet Neurol. 2014 Jan;13(1):67–82.
- Lenert P, Icardi M, Dahmoush L. ANA(+) ANCA(+) systemic vasculitis associated with the use of minocycline: Case-based review. Clin Rheumatol. 2013 Jul;32(7):1099–1106.
- Kermani TA, Ham EK, Camilleri MJ, Warrington KJ. Polyarteritis nodosa-like vasculitis in association with minocycline use: A single-center case series. Semin Arthritis Rheum. 2012 Oct;42(2):213–221.
- Thaisetthawatkul P, Sundell R, Robertson CE, Dyck PJ. Vasculitic neuropathy associated with minocycline use. J Clin Neuromusc Dis. 2011 Jun;12(4):231–234.
- Starr MR, Tillema J-M, Ytterberg SR, et al. Minocycline-induced vasculitis presenting as a third nerve palsy. J Neuropthalmol. 2019 Jun;39(2):240–241.
- Garg N, Altowaijri GH, Nesbit GM, et al. Minocycline-associated vasculitis of extracranial branches of vertebral arteries presenting as myelopathy. Neurol Neuroinnunol Neuroinflamm. 2014 May 15;1(1):e7.
- Ogawa N, Kawai H, Yamakawa I, et al. [Case of minocycline-induced vasculitic neuropathy] (article in Japanese). Rinsho Shinkeigaku. 2010 May;50(5):301–305.
- Katada Y, Harada Y, Azuma N, et al. Minocycline-induced vasculitis fulfilling the criteria of polyarteritis nodosa. Mod Rheumatol. 2006;16(4):256–259.
- Klaas JP, Matzke T, Makol A, Fulgham JR. Minocycline-induced polyarteritis nodosa-like vasculitis presenting as a brainstem stroke. J Clin Neurosci. 2015 May;22(5):904–907.
- Baratta JM, James P, Dyck B, et al. Vasculitic neuropathy following exposure to minocycline. Neurol Neuroimmunol Neuroinflamm. 2015 Nov 12;3(1):e180.
- McMillan HJ, Jansen GH, Koujok K, et al. Mononeuritis multiplex associated with minocycline in an adolescent. Muscle Nerve. 2017 Oct;56(4):E33–E35.
- Marzo-Ortega H, Baxter K, Strauss RM, et al. Is minocycline therapy in acne associated with antineutrophile cytoplasmic antibody positivity? A cross-sectional study. Br J Dermatol. 2007 May;156(5):1005–1009.
- Lightfoot RW Jr., Michel BA, Bloch DA, et al. The American College of Rheumatology 1990 criteria for the classification of polyarteritis nodosa. Arthritis Rheum. 1990 Aug;33(8):1088–1093.
Authors’ note: Special thanks to John Sherbeck, MD, Department of Pathology, St. Joseph Mercy Hospital, Ann Arbor, Mich.