A 67–year-old white woman with primary Raynaud’s phenomenon presented following a week of progressively worsening shortness of breath, dry cough and generalized malaise. An avid tennis player, she first noticed dyspnea while playing, but a few days later grew short of breath even at rest. She went to an urgent care center, where a computed tomography (CT) scan of her chest reportedly showed an atypical pneumonia. She tested negative for SARS-CoV-2 and was discharged on oral doxycycline therapy for suspected bacterial pneumonia.
However, her shortness of breath continued to progress to the point that she was unable to speak in complete sentences. This prompted a visit to the emergency department, where she was found to be in respiratory distress. She was tachypneic with a respiratory rate of 38 per minute and hypoxemic with oxygen saturation of 70% on room air, requiring 4L per minute of supplemental oxygen to improve her saturation to 92%.
A laboratory evaluation was significant for neutrophil-predominant leukocytosis 18.1×103/uL (reference range [RR]: 4.5–11.0×103/uL), normocytic anemia with hemoglobin 8.9 g/dL (RR: 11.5–15.5 g/dL) and platelet count 492×103/uL (RR:130–400×103/uL). No evidence was found of hemolytic anemia, iron, B12 or folate deficiency. Her ferritin level was 576 ng/mL (RR: 15–150 ng/mL), her C-reactive protein (CRP) was 341.3 mg/L (RR: <6 mg/L), and her erythrocyte sedimentation rate (ESR) was 113 mm/hour (RR: <20 mm/hour). Her prothrombin time and international normalized ratio and partial thromboplastin time were normal. Her D-dimer was 6,069 ng/mL (RR: ≤499 ng/mL) and fibrinogen was 576 mg/dL (RR: 170–400 mg/dL). Her creatinine kinase was 44 U/L (RR: 30–300 units/L) and aldolase was 9.9 U/L (RR: 0.1–8.0 units/L). Her lactic acid was 3.8 mmol/L (RR: 0.7–2.0 mmol/L). Troponin I was undetectable, and an electrocardiogram was normal. Her urinalysis was normal.
The nasopharyngeal viral respiratory polymerase chain reaction test, Legionella urine antigen test and sputum and blood cultures were negative.
A chest CT now showed diffuse, patchy bilateral groundglass opacities and interlobular septal thickening, with small areas of dense bilateral pulmonary consolidations, trace bilateral pleural effusions, a mildly dilated esophagus and mediastinal lymphadenopathy (see Figure 1A, opposite).
Due to increasing oxygen requirements and concern for further decompensation, she was transferred to the medical intensive care unit. Here, she was started on empiric treatment for community-acquired pneumonia with ceftriaxone and azithromycin. A repeat SARS-CoV-2 test was negative. A transthoracic echocardiogram (TTE) was normal.
Three days later, she had failed to improve on antibiotic therapy and had worsening hypoxemia, requiring a non-rebreather mask. Suspicion for COVID-19 remained high, but she tested negative for a third time.
Given her history of Raynaud’s phenomenon and unremarkable infectious work-up, an autoimmune etiology was considered. She was subsequently started on 1 mg/kg of intravenous methylprednisolonone on hospital day 4, after which a consult with a rheumatologist was obtained.
She denied any history of rash, mucosal ulcers, photosensitivity, alopecia, gastroesophageal reflux disease, dysphagia, muscle pain or weakness, recurrent ear infections, sinusitis, recurrent pneumonia, epistaxis, joint pain, swelling, morning stiffness or renal disease. Prior to symptom onset, she had been completely asymptomatic. With the exception of playing tennis three or four times a week, she had limited her outside activities due to the pandemic and denied any known sick contacts.
She had previously been evaluated by a rheumatologist in the 1990s for Raynaud’s phenomenon, and she lacked anti-nuclear antibodies (ANA) and rheumatoid factor (RF). She also lacked antibodies against double-stranded DNA (dsDNA), extractable nuclear antigens (ENA) and cyclic citrullinated peptides (CCP). For 20 years, she had never experienced any complications of Raynaud’s or symptoms to suggest a systemic autoimmune disease, and she had never required therapy.
Initially, given the abrupt onset of dyspnea, lack of symptoms other than uncomplicated Raynaud’s, absence of physical exam findings to suggest an autoimmune disease, previously negative serologies and normal TTE, the suspicion for an autoimmune process was low. However, her hypoxemia improved with steroids, which prompted a rheumatologic work-up.
During this time, she was also evaluated by a pulmonologist, who expressed concern for cryptogenic organizing pneumonia. A bronchoscopy was discussed, but not pursued because her oxygen requirements had decreased to 2L per minute by hospital day 6.
Given her marked improvement, she was discharged on 60 mg of prednisone and supplemental oxygen by hospital day 8. She was seen in our rheumatology clinic a few days later for follow-up labs, which revealed a markedly positive C-ANCA of 1:256 and anti-PR3 of >8 U (RR: <0.4 U). Her serologic evaluation was remarkable for a positive RF at 36 U/mL (RR: <15 U/mL), negative anti-MPO, ANA, anti-ENA, anti-dsDNA, anti-SCL-70, anti-centromere, anti-CCP, myositis panel, anti-RNA polymerase III, anti-beta 2 glycoprotein IgM and IgG, anti-cardiolipin IgM and IgG.
Based on serologies and pulmonary manifestations, she was diagnosed with granulomatosis with polyangiitis (GPA). She continued 60 mg of prednisone daily and was started on 375 mg/m2 of rituximab weekly for four weeks. She was gradually tapered to 20 mg prednisone daily by six weeks following her discharge. At that time her CRP was 2.1 mg/L and her ESR was 29 mm/hr. She was C-ANCA positive at 1:512 with anti-PR3 of 1.6. Her fatigue, cough and shortness of breath had completely resolved, and she no longer required supplemental oxygen.
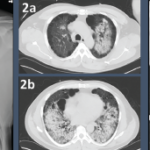
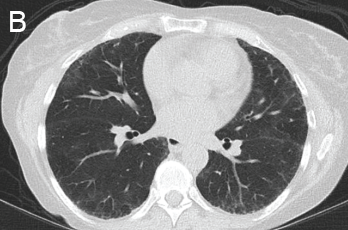
She was scheduled for a repeat CT two months post discharge, which showed dramatic improvement in the extensive bilateral infiltrates, moderate interstitial lung disease remaining more prominent in the lower lung fields, subpleural changes of obstructive pulmonary disease and mild air trapping in the lower lobes (see Figure 1B, opposite).
Introduction
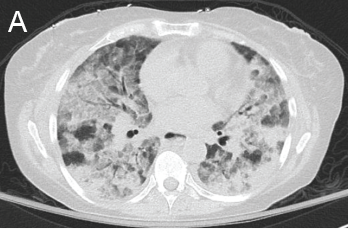
Figure 1: GPA radiographic progression
A novel coronavirus, designated SARS-CoV-2 by the World Health Organization, was identified at the end of 2019 after a large number of patients were diagnosed with pneumonia in Wuhan, China. The virus rapidly spread across the world, becoming a pandemic, with the first case in the U.S. reported on Jan. 20, 2020. The most common presenting symptoms include cough, fever, myalgia, dyspnea and pharyngitis. Presentation ranges from mild infection (~80%) to severe (~20%), in which patients experience respiratory failure. Severe illness predominantly occurs in adults of advanced age and in those with underlying medical conditions, but can also occur in otherwise healthy individuals.
A) Diffuse, patchy, bilateral groundglass opacities and interlobular septal thickening with small patches of dense pulmonary consolidation are seen.
B) Repeat CT after two doses of rituximab and steroids shows markedly improved groundglass opacities, resolved areas of consolidation and moderate residual subpleural interstitial lung disease.
Given the ongoing pandemic, the leading diagnosis considered for patients presenting to the hospital with respiratory symptoms is COVID-19. Often, bacterial and viral tests are run simultaneously, with most patients treated for bacterial pneumonia while awaiting results. The diagnosis of COVID-19 is made by direct detection of SARS-CoV-2 RNA by nucleic acid amplification tests (NAATs), most commonly reverse-transcription polymerase chain reaction (RT-PCR) from the upper respiratory tract.
The U.S. Food & Drug Administration has granted emergency use authorization for many different NAATs. The accuracy and predictive values of SARS-CoV-2 NAATs have not been systematically evaluated. Reported false negative rates have ranged from less than 5% to 40%, although these estimates are limited in part by the fact that no perfect reference standard for comparison exists. Given the high incidence of false negative tests, NAATs are often repeated if clinical suspicion for COVID-19 remains high. This has also led clinicians to rely on other diagnostic tools, such as chest imaging.
Here, we present a case of a previously healthy woman admitted with acute dyspnea on exertion, which raised suspicion for COVID-19 despite her first test being negative. CT imaging showed an impressive pattern of diffuse groundglass opacities with focal consolidations, which raised suspicion for a viral infectious etiology, specifically COVID-19. Because multiple bacterial and viral tests were negative, including three NAATs for SARS-CoV-2, and due to a lack of response to antibiotic treatment, other etiologies were considered.
The first concern was cryptogenic organizing pneumonia (COP) given her relatively acute presentation and CT findings. The other consideration was a potential autoimmune cause given her remote history of Raynaud’s. However, after evaluation by a rheumatologist, suspicion for autoimmune disease was lower, given the absence of other clinical features to suggest it. Nevertheless, serologic evaluation showed high titers of proteinase 3 (PR3) antibodies. The patient was, thus, diagnosed with GPA restricted to the lung based on isolated pulmonary manifestations, strongly positive serologies and response to high doses of steroids.
GPA is an uncommon disease characterized by a chronic granulomatous necrotizing vasculitis of mainly small- and medium-sized vessels. It affects three out of every 100,000 people in the U.S. The mean age at diagnosis is 50 years, and the majority (90%) of cases occur in the Caucasian population. GPA affects multiple organ systems and may involve any part of the body. It has a broad clinical spectrum, ranging from localized disease (predominantly restricted to the respiratory tract) to a severe life-threatening form with involvement of multiple organs (predominantly the kidneys and lungs). Patients typically present with weeks to months of constitutional symptoms and later develop signs or symptoms of specific organ involvement. Even though the majority of patients have an insidious course, some can have an explosive presentation over days, like our patient.
Serum cytoplasmic ANCA titer elevation occurs in 82–94% of GPA patients, depending on disease severity. It is primarily associated with anti-PR3, while microscopic polyangiitis is primarily associated with anti-myeloperoxidase (MPO). GPA antibody test results can take several days.
Clinical suspicion for this disease may be delayed in the current climate of a pandemic, especially if it occurs in the absence of other signs or symptoms to suggest an autoimmune etiology, such as in our patient, who presented with isolated pulmonary symptoms. Chest CTs can be readily available and provide some clues to help differentiate infectious from autoimmune etiologies, such as GPA. Here, we review the radiographic characteristic features of the three main diagnostic considerations in this patient: COVID-19 infection, GPA and COP.
CT Findings in COVID-19
Multiple studies have been recently published describing CT characteristics of COVID-19 infection. All of them suggest the proportion of CT abnormalities in patients infected with COVID-19 is very high. The most typical CT imaging finding is the presence of groundglass opacities, with more than half of the patients having some combination of groundglass opacities, consolidation and adjacent pleural thickening.
A meta-analysis of 13 studies with a total of 2,738 participants reported a pooled positive rate of CT imaging of 89.76% (95% CI 84.42–93.84%). The typical CT imaging appearance for COVID-19 was groundglass opacities (83.31%), groundglass opacities with mixed consolidation (58.42%), adjacent pleural thickening (52.46%), interlobular septal thickening (48.46%) and air bronchograms (46.46%). Other, less common CT signs included crazy paving pattern (14.81%), pleural effusion (5.88%), bronchiectasis (5.42%), pericardial effusion (4.55%) and lymphadenopathy (3.38%). The most common anatomic distribution is bilateral lung (78.2%; 95% CI: 65.69–88.19%). The lesions were mostly located in the periphery (76.95%; 95% CI: 57.43–91.50%) and lower lobes (65.22%; 95% CI: 55.95–73.94%).1
A study from four centers in China analyzed the CT findings in relationship to the time of symptom onset. They demonstrated that CT findings occurred more commonly in those with longer duration following symptom onset. Fifty-six percent of the patients imaged zero to two days after symptom onset had a normal CT compared with only 9% obtained between three and five days after time of onset and 4% between six and 12 days from symptom onset. The CT patterns were similar to those described in the meta-analysis above, with a preponderance of groundglass abnormalities in early disease followed by development of crazy paving and, finally, increasing consolidation later in the disease course.2
CT Findings in GPA
Fewer studies have described the typical CT findings in GPA. Pulmonary nodules and masses are the most common radiologic findings, seen in up to 70% of patients either at presentation or during the course of the disease. Diffuse groundglass opacities and consolidation occur in up to 50% of patients with GPA. The most common distributions of groundglass opacities and consolidations are bilateral perihilar and peribronchovascular. These findings can wax and wane through the course of the disease.
It is important to note that diffuse alveolar hemorrhage (DAH) can mimic these findings, especially because areas of hemorrhage can coalesce and appear as consolidations. A distinguishing feature of DAH would be groundglass opacities with sparing of the subpleural space.3,5
The prevalence of groundglass opacities and consolidations in GPA varies across the literature. Lohrmann et al. described the CT findings of 57 patients with GPA. In 51 patients (89%), the most commonly observed findings were nodules or masses, with a mean of five masses. They were bilateral in 37 patients (70%), and predominantly subpleural and diffuse. They represented isolated findings in 12 patients (23%) and were observed with other findings in 41 patients (77%). Fifteen patients (26%) had areas of groundglass attenuation and were associated with other abnormalities in 11 patients (72%).4
Here, we present a case of a previously healthy woman admitted with acute dyspnea on exertion, which raised suspicion for COVID-19 despite her first test being negative.
Because no specific or pathognomonic imaging findings exist to differentiate GPA from other conditions, suspicion for the disease should arise from clinical presentation and serologic markers.
Cryptogenic Organizing Pneumonia
COP, previously called bronchiolitis obliterans organizing pneumonia, is an interstitial disease that affects the distal airways, primarily bronchioles and alveoli. Disease onset occurs between the fifth and sixth decades of life, and it affects men and women equally. Patients present with non-specific symptoms of relatively short duration (weeks to months), such as fever, cough and dyspnea. There are three described imaging patterns of COP, but the typical pattern consists of patchy alveolar opacities ranging from groundglass opacities to consolidation with air bronchograms. These are usually bilateral and peripheral and may wax and wane just like in GPA. In contrast to other interstitial lung diseases, COP does not have honeycombing.6
Diagnosis of COP can be challenging since as the name suggests, the physician must ensure it is cryptogenic in origin and not secondary to an underlying disease. A long list of diseases and medications is associated with the typical histopathological features and imaging findings of COP. Therefore, a careful evaluation of the patient’s underlying diseases, environmental exposures, medication use, physical examination and ancillary tests is required to determine if the organizing pneumonia is cryptogenic in nature. Differentiating between lung-limited GPA and COP can be challenging, because both conditions are radiographically indistinguishable. Organizing pneumonia has also been described as a rare variant of GPA, which has similar clinical, radiographic and even histologic findings. It can sometimes be distinguished from GPA by the absence of extensive areas of geographic necrotizing granulomatous inflammation.7
Summary
Any patient presenting with respiratory symptoms in the current climate raises suspicion for COVID-19, but other etiologies should always be considered. Although our patient’s acute onset of respiratory symptoms with the presence of groundglass opacities and consolidations on imaging were concerning for COVID-19, a thorough evaluation of other potential etiologies, including GPA, cryptogenic organizing pneumonia, other idiopathic interstitial pneumonias, hypersensitivity pneumonitis and DAH, was performed to arrive at her diagnosis. Ultimately, the combination of multiple COVID-19 tests to rule out a false negative, the lack of response to antibiotics, positive serologies, a good response to steroids and CT findings helped rule out infection and establish the diagnosis of GPA.
 Andrea Ramirez-Gomez, MD, is a second-year rheumatology fellow at Washington University School of Medicine, St. Louis.
Andrea Ramirez-Gomez, MD, is a second-year rheumatology fellow at Washington University School of Medicine, St. Louis.
 Katherine Kougias Temprano, MD, is a rheumatologist with BJC Medical Group, Saint Louis.
Katherine Kougias Temprano, MD, is a rheumatologist with BJC Medical Group, Saint Louis.
References
- Bao C, Liu X, Zhang H, Li Y, Liu J. Coronavirus Disease 2019 (COVID-19) CT findings: A systematic review and meta-analysis. J Am Coll Radiol. 2020 Jun;17(6):701–709.
- Bernheim A, Mei X, Huang M, et al. Chest CT findings in Coronavirus Disease-19 (COVID-19): Relationship to duration of infection. Radiology. 2020 Jun;295(3):200463.
- Thompson GE, Specks U. Update on the management of respiratory manifestations of the antineutrophil cytoplasmic antibodies-associated vasculitides. Clin Chest Med. 2019 Sep;40(3):573–582.
- Lohrmann C, Uhl M, Kotter E, et al. Pulmonary manifestations of Wegener granulomatosis: CT findings in 57 patients and a review of the literature. Eur J Radiol. 2005 Mar;53(3):471–477.
- Martinez F, Chung JH, Digumarthy SR, et al. Common and uncommon manifestations of Wegener granulomatosis at chest CT: Radiologic-pathologic correlation. Radiographics. 2012 Jan–Feb;32(1):51–69.
- Cordier JF, Cottin V, Lazor R, Thivolet-Béjui F. Many faces of bronchiolitis and organizing pneumonia. Semin Respir Crit Care Med. 2016 Jun;37(3):421–440.
- Shalin K, Wajihuddin S, Hess M. Bronchiolotis obliterans-organizing pneumonia-like variant of granulomatosis with polyangiitis. Chest [Practice Management and Administration.] 2015 Oct;148(4 suppl): 878A.