Discussion
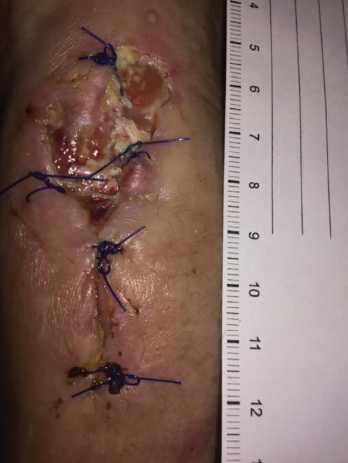
Figure 5. Increased purulent drainage from the left wrist in December 2018.
Mycobacterium kansasii is a slow-growing, photochromogenic, acid-fast nontuberculous mycobacterium (NTM) that was first described as a human pathogen in 1953.1,2 Even though the various NTM are common environmental organisms, M. kansasii has only been isolated from tap water in endemic areas, such as the southeastern and southern coastal states of the U.S.2,3 M. kansasii most typically causes pulmonary (i.e., upper lobe fibrocavity or chronic nodular) disease after organ transplantation, in patients with chronic lymphocytic leukemia and those infected by human immunodeficiency virus; however, it may also rarely manifest as skin and soft tissue, skeletal or disseminated disease.2
Disseminated cutaneous infections, characterized by multiple noncontiguous nodular draining skin lesions, are most commonly caused by rapidly growing NTM, such as M. chelonae and M. abscessus complex.2 These patients typically have underlying inflammatory diseases, such as rheumatoid arthritis, and are on chronic steroids, with doses as low as 5 mg of prednisone daily.4 Some slow-growing NTM, mostly commonly M. haemophilum and M. ulcerans, can cause a similar syndrome, but are typically in patients on higher dose steroids or multiple immunosuppressants.2
Biologics, especially anti-tumor necrosis factor (TNF) agents, have radically changed the treatment of inflammatory conditions. These agents have been associated with increased rates of reactivation of latent tuberculosis and de novo NTM infections, attributed to inhibition of the pro-inflammatory cytokine TNF involved in macrophage recruitment and formation of granulomas, which help contain and clear mycobacterial infections.4
Tofacitinib is a Janus kinase (JAK) inhibitor. JAKs are intracellular enzymes involved in signal transmission for cytokines. Tofacitinib primarily inhibits JAK1 and JAK3, and to some degree JAK2, which blocks the effects of interferon (IFN) γ, interleukin (IL) 6, IL-12 and IL-23; this inhibits the differentiation and action of T helper (Th) 1, Th2 and Th17 cells involved in adaptive and innate immune responses.5 Two cases have been reported of NTM pulmonary infections in rheumatoid arthritis patients receiving tofacitinib, in addition to other disease-modifying anti-rheumatic drugs (DMARDs), in a review of phase 2, 3 and long-term extension clinical trial data.6
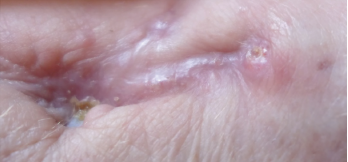
Figure 6. Close-up of the left wrist wound June 2019, with complete skin closure without further drainage.
Ustekinumab is a human IgG monoclonal antibody that binds the p40 protein subunit of IL-12 and IL-23. Both IL-12 and IL-23 are involved in the differentiation of CD4+ T cells to T helper (Th) lineage cells, Th1 cells (a source of IFN- γ) and Th17 cells (a source of IL-17 and TNF), which mediate cellular immunity.7 The risk of NTM infections, specifically M. kansasii, with the use of ustekinumab is unknown. A case was recently reported of an NTM infection (specifically M. abscessus) in a patient with Crohn’s disease treated with ustekinumab monotherapy, which had not been previously reported.8
Our patient was on dual biologic therapy for inflammatory bowel disease and seronegative inflammatory arthritis. Her reported duration of treatment with dual biologic therapy, methotrexate, hydroxychloroquine and prednisone before presentation was greater than 10 years. The use of dual biologic therapy is rare for inflammatory bowel disease and inflammatory arthritis, but has been used in cases where patients have refractory disease.9,10 These case series followed small numbers of patients for up to two years, with few reported adverse events.
A recent systematic review and meta-analysis of the use of two biologic DMARDs in rheumatoid arthritis did suggest an increased risk of serious infections and other adverse events.11 Currently, an open-label, phase 4 study is actively enrolling patients to assess triple therapy with vedolizumab, adalimumab and methotrexate in patients with newly diagnosed Crohn’s disease.10 It is unknown if similar studies looking at multiple biologic agents are underway for inflammatory arthritis patients.
Table 1: Initial Laboratory Data
| Variable | Result | Reference Range |
|---|---|---|
| White blood cell count | 7.9 X 103/μL | 4.0–9.5 X 103/μL |
| Hemoglobin | 11.3 gm/dL | 11.7–15.5 gm/dL |
| Hematocrit | 0.368 | 35.7–45.8% |
| Platelets | 303 X 103/μL | 145–357 X 103/μL |
| Sodium | 139 mmol/L | 135–145 mmol/L |
| Potassium | 3.5 mmol/L | 3.5–5.0 mmol/L |
| Chloride | 98 mmol/L | 98–107 mmol/L |
| Bicarbonate | 29 mmol/L | 22–31 mmol/L |
| Blood urea nitrogen | 18 mg/dL | 1–18 mg/dL |
| Creatinine | 0.67 mg/dL | 0.70–1.20 mg/dL |
| Glucose | 85 mg/dL | 65–199 mg/dL |
| C-reactive protein | 31.2 mg/L | ≤4.9 mg/L |
| Sedimentation rate | 32 mm/hr | 0–20 mm/hr |
Our patient had fallen on her hands before her move from Texas. Minor skin breakdown may have served as a point of inoculation that resulted in M. kansasii infection, given its prevalence in tap water in southern states.3 The concomitant use of methotrexate and prednisone with tofacitinib and ustekinumab likely increased her risk of more serious infection. Despite the number of immunosuppressive medications, our patient had very poor control of her inflammatory arthritis symptoms, yet was on a stable regimen for over 10 years without trials of other agents. Coordination between her specialists may have better targeted the organ systems affected by her inflammatory processes, resulting in a more favorable balance of infection risk and symptom control.


