The currently available evidence suggests an increased risk of Mycobacterium tuberculosis infection with the use of TNFi’s. However, the occurrence of non-tuberculous mycobacterial infections with the use of biologics is not well established.
The study of non-tuberculous mycobacterial diseases is a recent development. The diagnosis may be delayed given the atypical and subtle presentations of non-tuberculous mycobacterial infections.
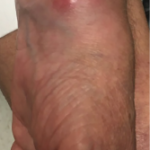
In our patient, reactivation of MAC in the lung in the setting of TNFi therapy led to disseminated disease with the active involvement of a single joint. This is an undescribed and uncommon finding as far as we were able to identify in the literature.
Many existing guidelines and the Centers for Disease Control and Prevention indicate the importance of screening all patients for latent Mycobacterium tuberculous infections before initiating a TNFi.8,9 Doing so involves taking a close history of tuberculosis risk factors and the use of at least one diagnostic test for tuberculosis (i.e., interferon-γ release assay or tuberculin skin test). Despite the health risks associated with non-tuberculous mycobacterial infections and its unpredictable presentation, screening for these infectious processes before initiating TNFi has not been standardized in practice.
Identifying the risk of non-tuberculous mycobacterial disease is challenging. During screening, physicians should consider obtaining sputum for culture if patients have a chronic unexplained cough or chest radiograph/computerized tomography scan suggestive of infiltrate or bronchiectasis.
Finally, pulmonary or infectious disease referral for confirmation of diagnosis should be considered.
 Bradley Bohman, MD, is a third-year post-graduate in the internal medicine residency program at the University of Arizona, Tucson.
Bradley Bohman, MD, is a third-year post-graduate in the internal medicine residency program at the University of Arizona, Tucson.
 Jawad Bilal, MBBS, is an assistant professor of medicine in the Division of Rheumatology at the University of Arizona, Tucson.
Jawad Bilal, MBBS, is an assistant professor of medicine in the Division of Rheumatology at the University of Arizona, Tucson.
References
- Zella S, Keiphof J, Haghikia A, et al. Successful therapy with rituximab in three patients with probable neurosarcoidosis. Ther Adv Neurol Disord. 2018 Oct 26;11:1756286418805732.
- Dobler CC. Biologic agents and tuberculosis. Microbiol Spectr. 2016 Dec; 4(6).
- Ai JW, Zhang S, Ruan QL, et al. The risk of tuberculosis in patients with rheumatoid arthritis treated with tumor necrosis factor-α antagonist: A metaanalysis of both randomized controlled trials and registry/cohort studies. J Rheumatol. 2015 Dec;42(12):2229–2237.
- Salt E, Wiggins AT, Rayens MK, et al. Risk factors for targeted fungal and mycobacterial infections in patients taking tumor necrosis factor inhibitors. Arthritis Rheumatol. 2016 Mar;68(3):597–603.
- Liao TL, Lin CH, Chen YM, et al. Different risk of tuberculosis and efficacy of isoniazid prophylaxis in rheumatoid arthritis patients with biologic therapy: A nationwide retrospective cohort study in Taiwan. PLoS One. 2016 Jun;11(4):e0153217.
- Lim CH, Chen HH, Chen YH, et al. The risk of tuberculosis disease in rheumatoid arthritis patients on biologics and targeted therapy: A 15-year real world experience in Taiwan. PLoS One. 2017 Jun 1;12(6):e0178035.
- Winthrop KL, Baxter R, Liu L, et al. Mycobacterial diseases and antitumour necrosis factor therapy in USA. Ann Rheum Dis. 2013 Jan;72(1):37–42.
- Diel R, Hauer B, Loddenkemper R, Manger B, Krüger K. [Recommendations for tuberculosis screening before initiation of TNF-α-inhibitor treatment in rheumatic diseases.] [article in German.] Z Rheumatol. 2009 Jul;68(5):411–416.
- Centers for Disease Control and Prevention (CDC). Tuberculosis associated with blocking agents against tumor necrosis factor-α—California, 2002–2003. MMWR Morb Mortal Wkly Rep. 2004 Aug 6;53(30):683–686.