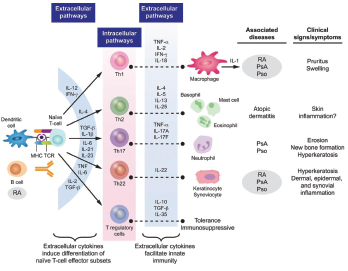
Figure 3: Cytokine Involvement in Psoriatic Arthritis6
In dendric cells and monocytes, phosphodiesterase inhibition increases intracellular cyclic AMP, activating protein kinase A (PKA). PKA leads to phosphorylation of several transcription factors altering transcriptional activity [inhibiting NFĸB transcriptional activity and reducing its subsequent expression of inflammatory cytokines (TNFα and IL-23) and stimulation of CREB (cAMP response element-binding protein) with increasing IL-10 expression]. The alteration of cytokines is believed to work downstream to decrease inflammation and proliferation in the synovium and keratocytes.
Case Presentation
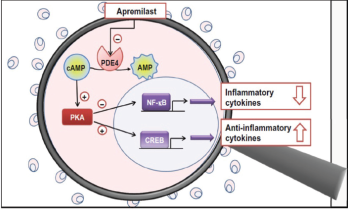
Figure 4: Proposed Mechanism of Apremilast
Our patient is a 71-year-old man with a history of psoriatic arthritis, psoriasis, aortic valve replacement (2015), maxillary osteotomy (1983) and tonsillectomy (1952), who presented with opportunistic Streptococcus salivarius bacteremia while on apremilast. The patient was initially diagnosed with psoriatic arthritis in 1981.
His medication history included multiple non-steroidal anti-inflammatory drugs (NSAIDs), methotrexate (two or three years) and adalimumab (starting in 1995). Adalimumab was discontinued at the patient’s request because he was concerned about potential cardiovascular side effects. He was switched to apremilast on June 10, 2015.
Five months after starting apremilast, he had a prosthetic aortic valve replacement for symptoms that predated the medication’s initiation. The procedure was performed without incident. The patient remained on apremilast with no noted toxicities or side effects until three years later.
With no provoking factors, the patient developed a low-grade fever and chills in July 2018. Blood cultures were obtained, and he was found to have S. salivarius bacteremia, a known opportunistic pathogen.6
An evaluation for an alternative etiology of immunosuppression was negative. He was placed on intravenous ceftriaxone from July 25, 2018, through Sept. 7, 2018. A transesophageal echocardiogram (TEE) and a transthoracic echocardiogram (TTE) were both unremarkable. Apremilast was withheld while the patient was treated for bacteremia.
This potential side effect—the development of an opportunistic infection while taking apremilast—was reported to the U.S. Food & Drug Administration (FDA) via MedWatch.
Discussion
S. salivarius is an anaerobic, gram-positive bacteria that colonizes the oral mucosa of humans. It is part of the larger group of Streptococcus viridans. S. viridans are known for their ability to synthesize dextrans from glucose, and their associated components of fibronectin binding protein/teichoic acid. These features allow them to adhere to fibrin-platelet aggregates, such as artificial heart valves, and accounts for their increased pathogenicity in subacute bacterial endocarditis.10,11 The S. salivarius bacteria reside outside the oral mucosa only in immunocompromised hosts as an opportunistic infection.10,13 It is also demonstrated in the literature to be associated with infective endocarditis in the immunocompromised host.12,14,15 This case identifies such an infection in a patient on apremilast.
Bacteremia with an opportunistic S. viridans in a patient with a history of a prosthetic valve replacement warranted full treatment for infective endocarditis using Dukes criteria. Our patient had two major criteria: positive persistent blood cultures with a typical microorganism for infectious endocarditis (S. viridans), and the infection was persistent with blood cultures drawn more than 12 hours apart. Although the TTE and TEE were unremarkable, he also had the minor criteria of a predisposing heart condition (a prosthetic valve) and a fever of greater than 38ºC (100.4ºF). Bioprosthetic valves carry a greater risk for infection than mechanical valves after the first 18 months. The risk of late prosthetic valve endocarditis involving the streptococcus species is also highest 12 months after valve implantation. Both of these trends, however, are demonstrated in non-opportunistic infections in immunocompetent patients.10,11
Review of the FDA public dashboard for the Adverse Event Reporting System demonstrates no cases are currently listed as an opportunistic infection for apremilast.16 It does report 19 cases of Streptococcus bacteremia and 18 cases of Streptococcal endocarditis since 2019. There is no specification of the species of Streptococcus provided. Other infections that may be considered opportunistic, but are not clearly specified by an opportunistic category are: six cases of oral candidiasis, 16 cases of cytomegalovirus infection, 17 cases of progressive multifocal leukoencephalopathy, 11 cases of brain abscesses, 11 cases of pulmonary mycosis, 15 cases of hepatitis B viral reactivation and 72 cases of active tuberculosis. There may be an underrepresentation based on categorization of underlying risk of infection from immunosuppression.
Biologic medications used in the treatment of psoriatic arthritis that alter the same inflammatory cytokine milieu as apremilast, including TNF, IL-17 and IL-12/23 and JAK inhibitors, are known immunosuppressive medications. Case reports of infective endocarditis are documented mainly for TNF inhibitors, but also IL-12/23 inhibitors.17,18 The FDA Adverse Event Reporting System has reports of endocarditis for all biologic subcategories, and the package inserts of all these biologics also include a warning regarding an increased risk of opportunistic infection.16