Apremilast was first marketed in March 2014 for the treatment of adults with psoriatic arthritis (PsA). An immunomodulating drug, which is a small molecule inhibitor of phosphodiesterase 4 (PDE4) specific for cyclic adenosine monophosphate (cAMP), apremilast is administered orally. By inhibiting PDE4, intracellular cAMP levels are increased.
Although the exact mechanism of action is not known, the Psoriatic Arthritis Long-Term Assessment of the Clinical Efficacy (PALACE) study evaluation of biomarkers revealed an increase in the anti-inflammatory mediator interleukin (IL) 10 and a decrease in pro-inflammatory mediators, tumor necrosis factor-α (TNFα), IL-17A and IL-23. Studies based on biopsies from active psoriatic synovial and skin lesions demonstrates these are the key cytokines involved in the pathogenesis of psoriatic arthritis.1,2
The PALACE study demonstrates a non-significant, 0.6 and 0.0 exposure-adjusted incidence rate/100 patient-years of opportunistic infection in placebo vs. apremilast, respectively.3 The ACTIVE trial produced consistent safety profile results.4 Even though no increased risk of opportunistic infections was identified, alterations in inflammatory cytokine levels were observed. This alteration of the inflammatory milieu may have immunosuppressive effects (see Figures 1 & 2, & 3).
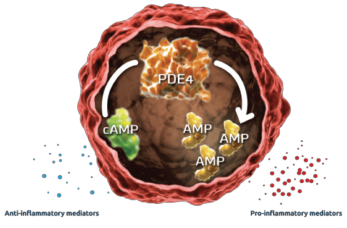
Figure 1: Mechanism of PDE45
Based on the PALACE study, the apremilast package insert does not mention an increased risk of opportunistic infection associated with the use of this drug.
In PsA, activated T cells and macrophages induce production of inflammatory cytokines and chemokines. The key cytokines in inducing the clinical features of psoriatic arthritis are IL-17, TNFα and IL-12/23. IL-17A is a proinflammatory cytokine produced by T helper 17 cells, macrophages, mast cells, dendritic cells, natural killer (NK) cells and CD8 T cells.
IL-17 is a key cytokine in the pathogenesis of PsA. Synovial biopsies from patients with PsA demonstrate higher levels of IL-17 than similar biopsies from patients with rheumatoid arthritis (RA).6 IL-17 works in targeted tissues including the keratinocyte (leading to epidermotropism of T cells and neutrophils and a subsequent psoriatic plaque), synovial fibroblast (with proliferation of the synovial fibroblast and induction of metalloproteinases that break down bone), endothelial cell (inducing angiogenesis and, paired with the activated synovial fibroblast, leading to inflammatory pannus of the synovium) and osteoclast (activating the RANK system and leading to osteoclastogenesis and formation of bone erosions).7
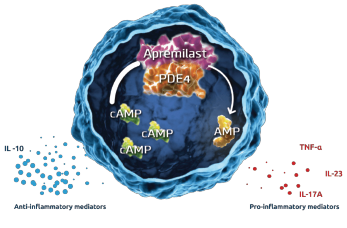
Figure 2: Inhibition of PDE4 by Apremilast5
TNFα is a proinflammatory cytokine overexpressed in the synovium, leading to synovial hyperproliferation, increased T cell and macrophage infiltration, induction of angiogenesis, osteoclast activation, synovial proliferation and metalloprotease release leading to joint destruction.8
IL-12 is produced by monocytes and macrophages in response to inflammation. Its activation results in stimulation and differentiation of Th1 cells, which leads to increased macrophages and triggering of a cellular immunity response.
IL-23 induces Th17 differentiation and subsequent increase of IL-17 and IL-22, producing subsequent downstream effects (including activation and inflammatory reaction synovio-entheseal complex and resulting in collagen enthesitis.)6
IL-22 acts on keratinocytes and synoviocytes leading to hyperkeratosis and dermal, epidermal and synovial inflammation (see Figure 4).
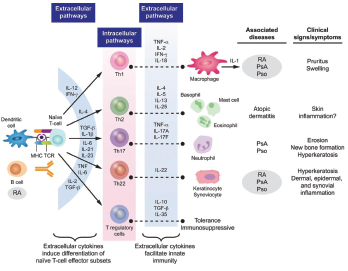
Figure 3: Cytokine Involvement in Psoriatic Arthritis6
In dendric cells and monocytes, phosphodiesterase inhibition increases intracellular cyclic AMP, activating protein kinase A (PKA). PKA leads to phosphorylation of several transcription factors altering transcriptional activity [inhibiting NFĸB transcriptional activity and reducing its subsequent expression of inflammatory cytokines (TNFα and IL-23) and stimulation of CREB (cAMP response element-binding protein) with increasing IL-10 expression]. The alteration of cytokines is believed to work downstream to decrease inflammation and proliferation in the synovium and keratocytes.
Case Presentation
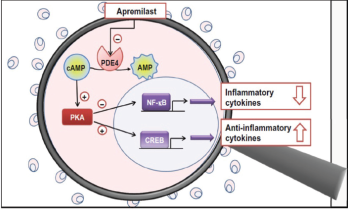
Figure 4: Proposed Mechanism of Apremilast
Our patient is a 71-year-old man with a history of psoriatic arthritis, psoriasis, aortic valve replacement (2015), maxillary osteotomy (1983) and tonsillectomy (1952), who presented with opportunistic Streptococcus salivarius bacteremia while on apremilast. The patient was initially diagnosed with psoriatic arthritis in 1981.
His medication history included multiple non-steroidal anti-inflammatory drugs (NSAIDs), methotrexate (two or three years) and adalimumab (starting in 1995). Adalimumab was discontinued at the patient’s request because he was concerned about potential cardiovascular side effects. He was switched to apremilast on June 10, 2015.
Five months after starting apremilast, he had a prosthetic aortic valve replacement for symptoms that predated the medication’s initiation. The procedure was performed without incident. The patient remained on apremilast with no noted toxicities or side effects until three years later.
With no provoking factors, the patient developed a low-grade fever and chills in July 2018. Blood cultures were obtained, and he was found to have S. salivarius bacteremia, a known opportunistic pathogen.6
An evaluation for an alternative etiology of immunosuppression was negative. He was placed on intravenous ceftriaxone from July 25, 2018, through Sept. 7, 2018. A transesophageal echocardiogram (TEE) and a transthoracic echocardiogram (TTE) were both unremarkable. Apremilast was withheld while the patient was treated for bacteremia.
This potential side effect—the development of an opportunistic infection while taking apremilast—was reported to the U.S. Food & Drug Administration (FDA) via MedWatch.
Discussion
S. salivarius is an anaerobic, gram-positive bacteria that colonizes the oral mucosa of humans. It is part of the larger group of Streptococcus viridans. S. viridans are known for their ability to synthesize dextrans from glucose, and their associated components of fibronectin binding protein/teichoic acid. These features allow them to adhere to fibrin-platelet aggregates, such as artificial heart valves, and accounts for their increased pathogenicity in subacute bacterial endocarditis.10,11 The S. salivarius bacteria reside outside the oral mucosa only in immunocompromised hosts as an opportunistic infection.10,13 It is also demonstrated in the literature to be associated with infective endocarditis in the immunocompromised host.12,14,15 This case identifies such an infection in a patient on apremilast.
Bacteremia with an opportunistic S. viridans in a patient with a history of a prosthetic valve replacement warranted full treatment for infective endocarditis using Dukes criteria. Our patient had two major criteria: positive persistent blood cultures with a typical microorganism for infectious endocarditis (S. viridans), and the infection was persistent with blood cultures drawn more than 12 hours apart. Although the TTE and TEE were unremarkable, he also had the minor criteria of a predisposing heart condition (a prosthetic valve) and a fever of greater than 38ºC (100.4ºF). Bioprosthetic valves carry a greater risk for infection than mechanical valves after the first 18 months. The risk of late prosthetic valve endocarditis involving the streptococcus species is also highest 12 months after valve implantation. Both of these trends, however, are demonstrated in non-opportunistic infections in immunocompetent patients.10,11
Review of the FDA public dashboard for the Adverse Event Reporting System demonstrates no cases are currently listed as an opportunistic infection for apremilast.16 It does report 19 cases of Streptococcus bacteremia and 18 cases of Streptococcal endocarditis since 2019. There is no specification of the species of Streptococcus provided. Other infections that may be considered opportunistic, but are not clearly specified by an opportunistic category are: six cases of oral candidiasis, 16 cases of cytomegalovirus infection, 17 cases of progressive multifocal leukoencephalopathy, 11 cases of brain abscesses, 11 cases of pulmonary mycosis, 15 cases of hepatitis B viral reactivation and 72 cases of active tuberculosis. There may be an underrepresentation based on categorization of underlying risk of infection from immunosuppression.
Biologic medications used in the treatment of psoriatic arthritis that alter the same inflammatory cytokine milieu as apremilast, including TNF, IL-17 and IL-12/23 and JAK inhibitors, are known immunosuppressive medications. Case reports of infective endocarditis are documented mainly for TNF inhibitors, but also IL-12/23 inhibitors.17,18 The FDA Adverse Event Reporting System has reports of endocarditis for all biologic subcategories, and the package inserts of all these biologics also include a warning regarding an increased risk of opportunistic infection.16
Bottom Line
It is likely the combination of a structurally abnormal valve and immunosuppression made the patient susceptible to valvular infection with an opportunistic organism. Infective endocarditis has severe morbidity and mortality. In the U.S. between 1980 and 2014, the mortality rate for endocarditis was 2.4 per 100,000 with an in-hospital mortality rate of around 20%.19 Contrary to previous thought, the decrease in pro-inflammatory mediators TNFα, IL-17 and IL-23 with apremilast may create immunosuppressant effects. These effects can have severe adverse outcomes, as in our patient. Caution should be used and further studies should be performed in this area.
 Stephanie Kydd Dondero, DO, is the chief fellow of the rheumatology program at Larkin Community Hospital/Lake Erie College of Osteopathic Medicine (LECOM), Miami.
Stephanie Kydd Dondero, DO, is the chief fellow of the rheumatology program at Larkin Community Hospital/Lake Erie College of Osteopathic Medicine (LECOM), Miami.
 Barry Waters, MD, has been in private practice in rheumatology in South Florida since 1986. For the past several years, he has served as the Rheumatology Fellowship program director for Larkin Community Hospital, Miami.
Barry Waters, MD, has been in private practice in rheumatology in South Florida since 1986. For the past several years, he has served as the Rheumatology Fellowship program director for Larkin Community Hospital, Miami.
References
- Partsch G, Steiner G, Leeb BF, et al. Highly increased levels of tumor necrosis factor-alpha and other proinflammatory cytokines in psoriatic arthritis synovial fluid. J Rheumatol. 1997 Mar;24(3):518–523
- van Kuijk AW, Reinders-Blankert P, Smeets TJ, et al. Detailed analysis of the cell infiltrate and the expression of mediators of synovial inflammation and joint destruction in the synovium of patients with psoriatic arthritis: Implications for treatment. Ann Rheum Dis. 2006 Dec;65(12):1551–1557.
- Edwards CJ, Blanco FJ, Crowley J, et al. Apremilast, an oral phosphodiesterase 4 inhibitor, in patients with psoriatic arthritis and current skin involvement: A phase III, randomized, control trial (PALACE 3). Ann Rheum Dis. 2016 Jun;75(6):1065–1073.
- Nash P, Ohson K, Walsh J, et al. Early and sustained efficacy with apremilast monotherapy in biological-naive patients with psoriatic arthritis: A phase IIIB, randomized controlled trial (ACTIVE). Ann Rheum Dis. 2018 May;77(5): 690–698.
- Otezla (apremilast). Psoriatic arthritis. Mechanism of action.
- Merola JF, Espinoza LR, Fleischmann R. Distinguishing rheumatoid arthritis from psoriatic arthritis. RMD Open. 2018 Aug 13;4(2):e000656.
- Menon B, Gullick NJ, Walter GJ, et al. Interleukin-17+CD8+ T cells are enriched in the joints of patients with psoriatic arthritis and correlate with disease activity and joint damage progression. Arthritis Rheumatol. 2014 May;66(5):1272–1281.
- Ritchlin CT, Haas-Smith SA, Li P, et al. Mechanisms of TNF-alpha- and RANKL-mediated osteoclastogenesis and bone resorption in psoriatic arthritis. J Clin Invest. 2003 Mar;111(6):821–831.
- Cauli A, Porru G, Piga M, et al. Clinical potential of apremilast in the treatment of psoriatic arthritis. Immunotargets Ther. 2014 Jun;2014(3):91–96.
- Doem CD, Burnham CA. It’s not easy being green: The viridans group streptococci, with a focus on pediatric clinical manifestations. J Clin Microbiol. 2010 Nov;48(11):3829–3835.
- Westling K. Viridans Group Streptococci septicaemia and endocarditis. Molecular diagnostics, antibiotic susceptibility and clinical aspects. Karolinska University Hospital, Huddinge. Karolinska Institutet, Stockholm, Sweden. 2005.
- Delorme C, Abraham AL, Renault P, Guédon E. Genomics of Streptococcus salivarius, a major human commensal. Infect Genet Evol. 2015 Jul;33:381–392.
- Ruoff KL, Miller SI, Garner CV, et al. Bacteremia with Streptococcus bovis and Streptococcus salivarius: clinical correlates of more accurate identification of isolates. J Clin Microbiol. 1989 Feb;27(2):305–308.
- Habib G, Hoen B, Tornos P, et al. Guidelines on the prevention, diagnosis, and treatment of infective endocarditis (new version 2009): The Task Force on the Prevention, Diagnosis, and Treatment of Infective Endocarditis of the European Society of Cardiology (ESC). Endorsed by the European Society of Clinical Microbiology and Infectious Diseases (ESCMID) and the International Society of Chemotherapy (ISC) for Infection and Cancer. Eur Heart J. 2009 Oct;30(19):2369–2413.
- Felix L, Gurunathan R. ‘I Can’t Believe It’s Not Bovis’: A case of Streptococcus salivarius related endocarditis (abstract 412). J Hosp Med. 2014;9(suppl 2).
- FDA Adverse Event Reporting System (FAERS) Public Dashboard. U.S. Food & Drug Administration. Current as of 2018 Jul 23.
- Mizuno R, Kiyosawa J, Fukuda A, et al. Infective endocarditis following tumor necrosis factor-α antagonist therapy for management of psoriatic erythroderma: A case report. J Med Case Rep. 2017 Feb 9;11(1):35
- Joost I, Steinfurt J, Meyer PT, et al. Staphylococcus aureus bacteremia with iliac artery endarteritis in a patient receiving ustekinumab. BMC Infect Dis. 2016 Oct 20;16(1):586.
- Wallace SM, Walton BI, Kharbanda RK, et al. Mortality from infective endocarditis: Clinical predictors of outcome. Heart. 2002 Jul;88(1):53–60.