After 13 months of treatment with sulfasalazine, we had to stop the medication due to drug-induced transaminitis. The patient’s psoriasis and arthritis worsened with etanercept monotherapy.
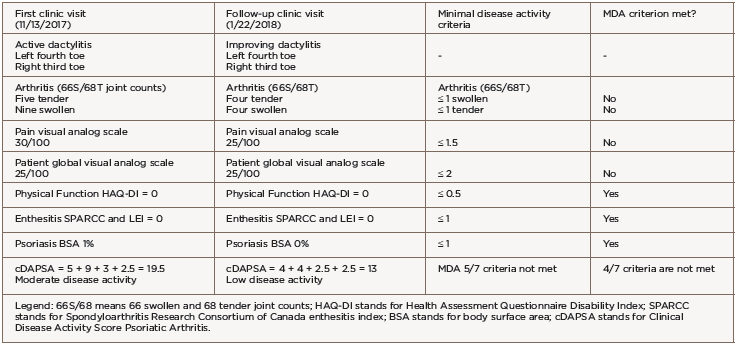
She was transitioned to certolizumab pegol 200 mg subcutaneous every 14 days with adequate disease control. However, after 12 months of therapy, she developed breakthrough peripheral arthritis with a clinical disease activity psoriatic arthritis score (DAPSA) of 19.5 (corresponding to moderate disease activity), scalp and genital psoriasis (on a total body surface area of 1%), as well as acute left fourth toe and right third toe dactylitis (see Table 1).
The patient did not meet PsA minimal disease activity criteria (MDA) due to five tender and nine swollen joints, a pain score of 30/100 and a patient global assessment score of 25/100. Given the failure of three tumor necrosis factor (TNF) inhibitors, we switched treatment to 90 mg of subcutaneous ustekinumab every 12 weeks, with loading doses at Days 0 and 28.
We evaluated the patient in our clinic for follow-up six weeks after the initial ustekinumab dose. Her psoriasis and peripheral arthritis had resolved. She demonstrated clinical improvement of the dactylitis and decreased erythema, swelling and tenderness (see Table 2). The patient’s clinical DAPSA was now 13, corresponding to low disease activity, but her PsA activity again did not meet MDA criteria due to four swollen/tender joints of two dactylitic toes, a pain score of 25/100 and a patient global assessment score of 25/100 (see Table 1).
Musculoskeletal ultrasound of her feet revealed hypoechoic collections surrounding the extensor tendons of these dactylitic digits as well as at the interphalangeal joints with Doppler enhancement, consistent with tenosynovitis and synovitis (see Figure 3). We saw no erosions. Radiographs showed slight spurring of the first metatarsophalangeal (MTP) joints and were otherwise unremarkable (see Figure 4). We continued ustekinumab and treated persistent toe dactylitis with local corticosteroid injections.
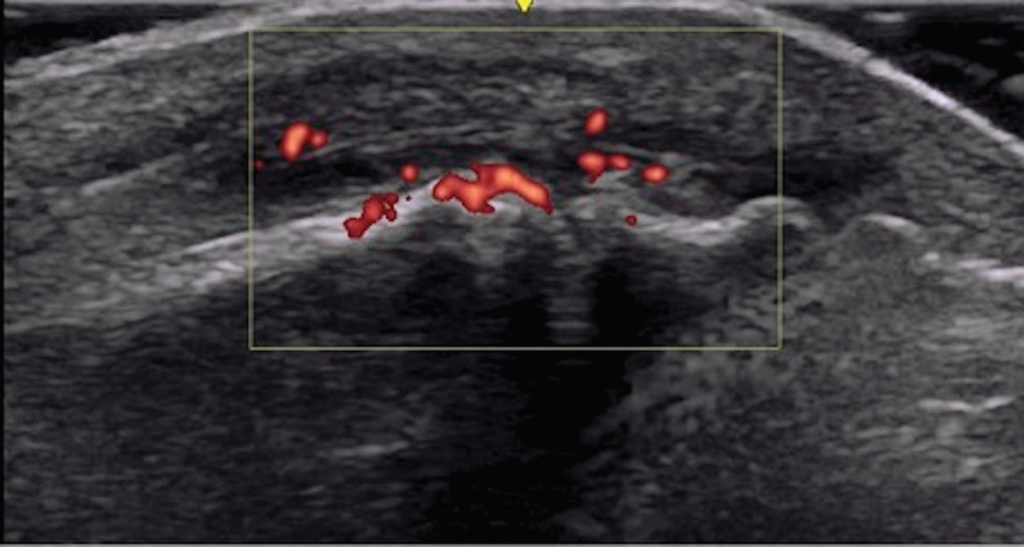
Figure 3. Interphalangeal (IP) toe joint with extensor tenosynovitis and IP synovitis with Doppler enhancement.
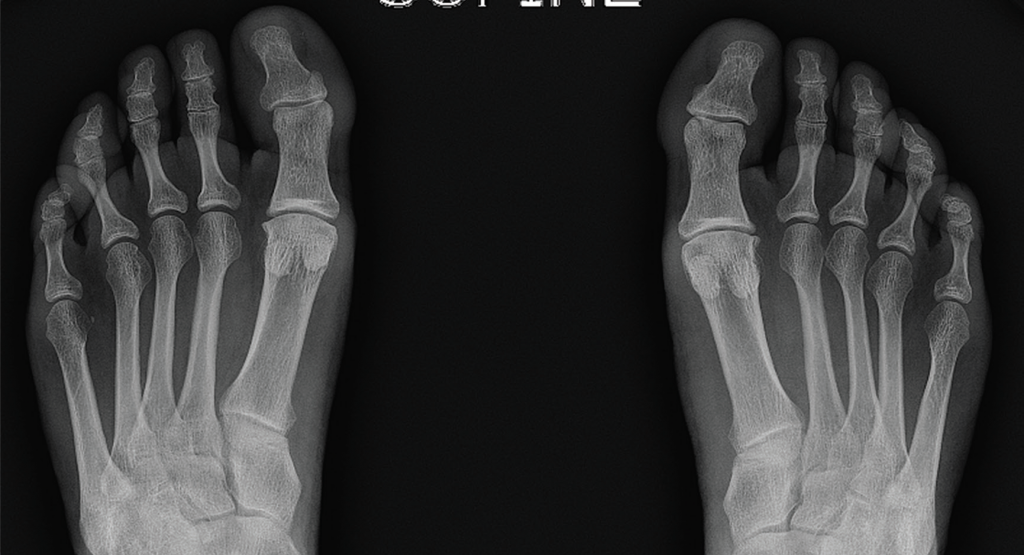
Figure 4. Bilateral feet radiographs appear normal except for subtle spurring at the bilateral first proximal interphalangeal joints.
Should ustekinumab augmented by local corticosteroid injection fail to adequately control disease after the third 90 mg dose (at 16 weeks of treatment), we plan to switch to a biologic with a different mechanism of action.