Discussion
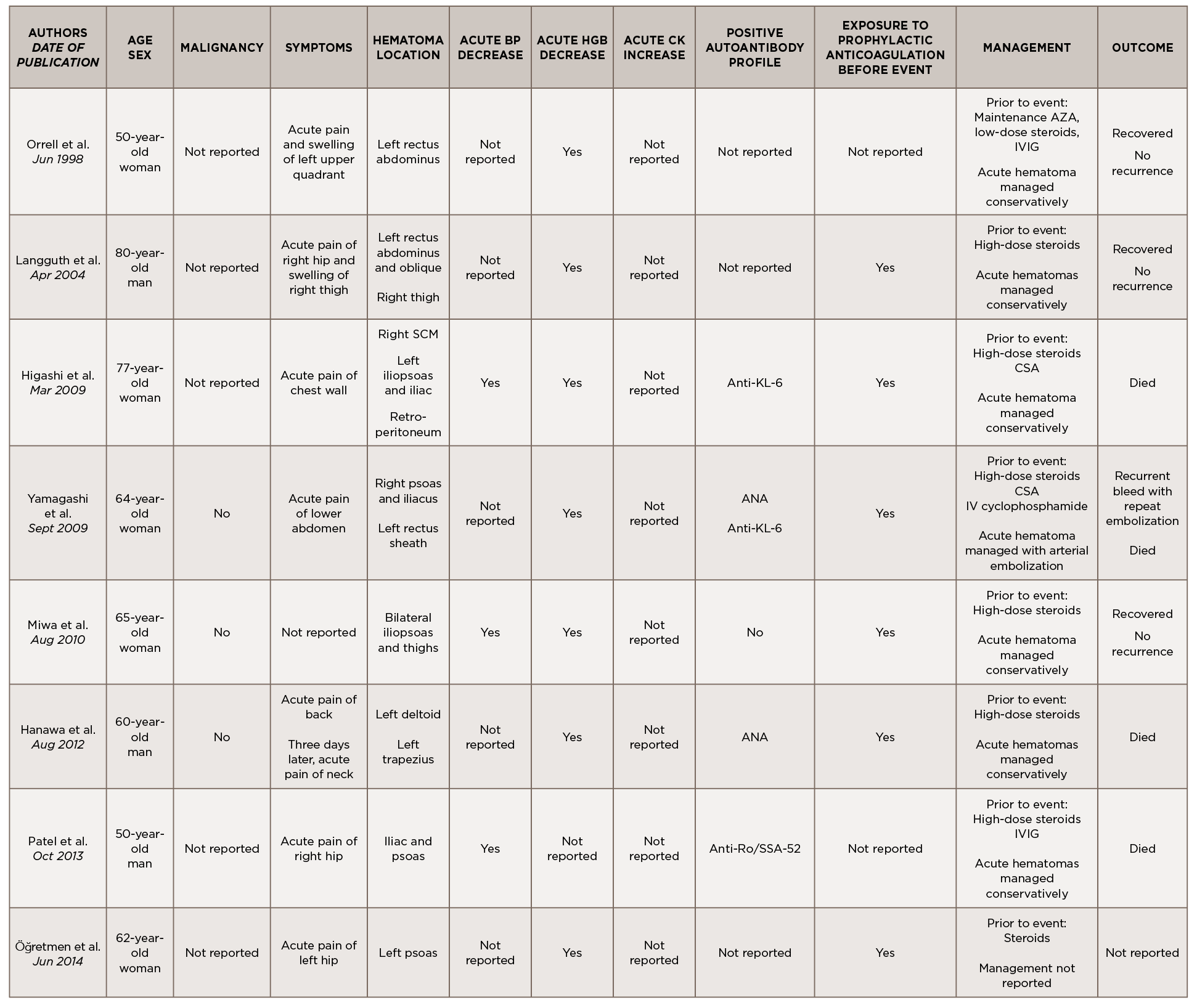
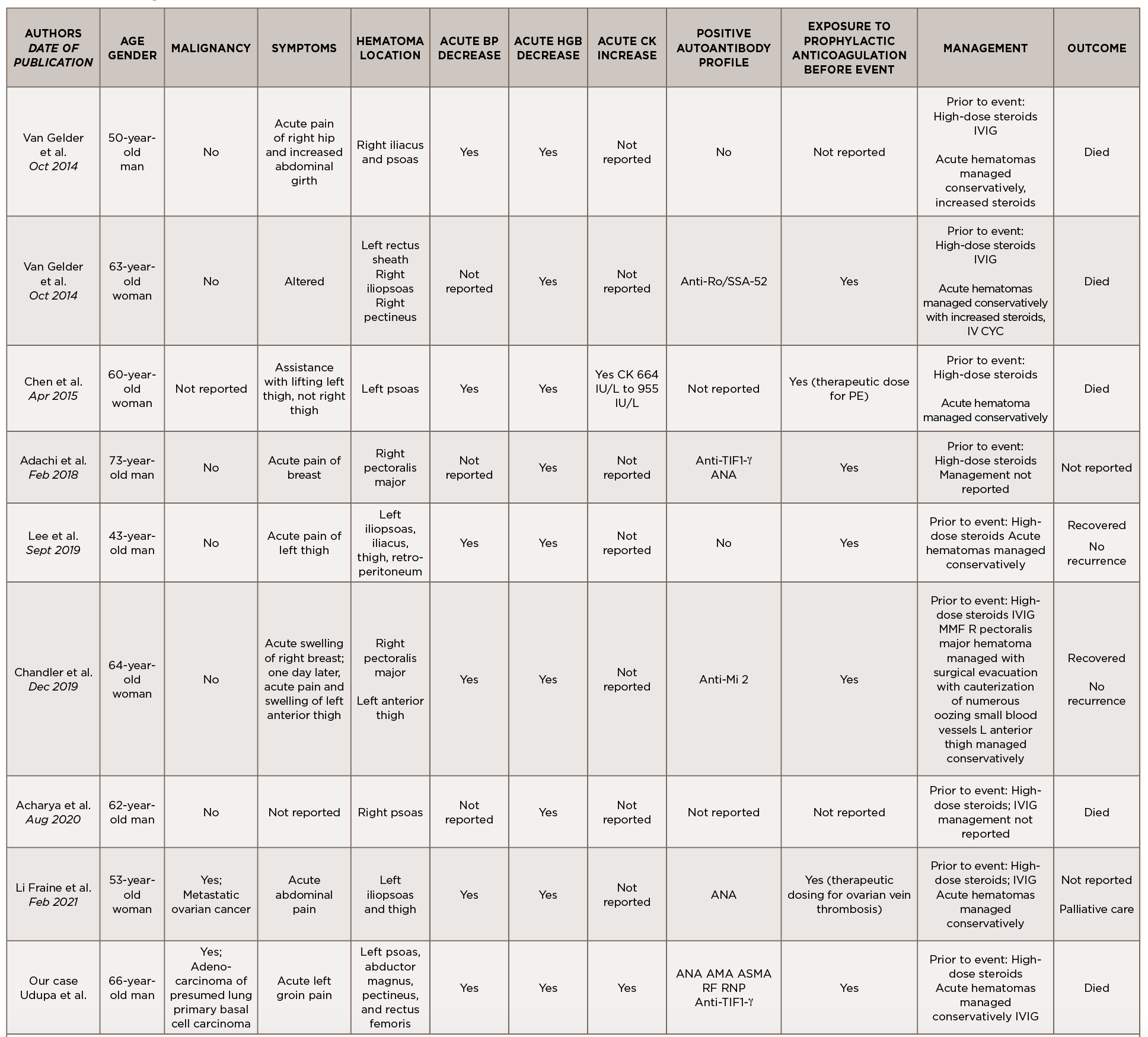
This is a case of anti-TIF1-γ positive paraneoplastic acute hemorrhagic dermatomyositis, an uncommon yet severe complication of dermatomyositis. As shown in Table 1, at least 16 adult cases of acute hemorrhagic dermatomyositis have been reported.4-18 The majority of these patients were middle aged, with approximately equal distribution among men and women.
Table 1
Legend: ANA, anti-nuclear antibody; AMA, anti-mitochondrial antibody; ASMA, anti-smooth muscle antibody; Anti-TIF1-γ, anti-transcription intermediary factor 1-γ; AZA, azathioprine; BP, blood pressure; CK, creatine kinase; CSA, cyclosporin A; CYC, cyclophosphamide; Hgb, hemoglobin; IVIG, intravenous immunoglobulin; MMF, mycophenolate mofetil; RF, rheumatoid factor; RNP, ribonucleoprotein; SCM, sternocleidomastoid; VS, vital signs
Although the past medical histories and clinical contexts differ slightly among these patients, only one was reported as having a concurrent malignancy.18 Most were newly diagnosed with dermatomyositis and subsequently developed atraumatic painful intramuscular hematomas, some of which were hemodynamically significant. All events occurred following administration of systemic glucocorticoids, and most events occurred following prophylactically or therapeutically dosed anticoagulation.
Some patients were treated with IVIG either before or after the hemorrhagic event, and a few were treated with disease-modifying anti-rheumatic drugs (DMARDs) either before, during or after acute hematoma formation.
Comprehensive autoimmune serologies were either not performed or not described in a majority of these case reports; of those reported, a positive ANA was seen in four patients, Sjögren’s syndrome-related antigen A (SSA) 52 kDa antibody in two patients, and Krebs von den Lungen 6 (KL-6) antibody in two patients. Apart from our case, only two other reports identified the presence of myositis-specific autoantibodies.14,16
Even with prompt recognition, acute hemorrhagic myositis can cause abrupt hemodynamic collapse and rapid progression to death. Aside from the inherent microvasculopathy of dermatomyositis, nearly all patients reported in the literature received either prophylactic or therapeutic doses of anticoagulation ahead of having a hemorrhagic event; this finding underscores the complex safety concerns surrounding anticoagulation in often immobile patients with underlying malignancies.
All patients reported in the literature seem to have developed hematomas while receiving systemic glucocorticoids and often did not respond to an escalation in steroid dose, calling into question the utility of immunosuppression in this clinical context.
Apart from vital sign changes and a decline in hemoglobin, the earliest diagnostic clue appears to be the acute onset of localized pain, which can be out of proportion to exam.
Apart from vital sign changes and a decline in hemoglobin, the earliest diagnostic clue appears to be the acute onset of localized pain, which can be out of proportion to exam. As in our case, a rapid rise in CK could also herald the presence of an intramuscular hematoma. Further research is needed to clarify whether the presence of certain autoantibodies, especially myositis-specific autoantibodies, confer a higher risk of developing hemorrhagic complications; this ultimately may help further define the clinical phenotypes associated with myositis-specific autoantibodies and aid in timely recognition and treatment.
In conclusion, acute hemorrhagic dermatomyositis is an unexpected yet potentially fatal complication of dermatomyositis. Further study is needed to understand its pathogenesis, identify those at highest risk, recognize early diagnostic clues and establish effective treatments.