Figure 3B: Hematoxylin and eosin stain demonstrates small- to medium-sized vessels that show necrotizing, fibrinoid necrosis representing a necrotizing vasculitis (arrows) (200x).
Figure 3C: Strongly positive amyloid β stain in the wall of subarachnoid blood vessels (100x).
Figure 3D: Salmon-colored tinge of amyloid on the Congo red stain (arrows) (400x). (Click to enlarge.)[/caption]
Cerebral amyloid angiopathy is a vasculopathy characterized by amyloid β (Aβ) deposition in the cortical and leptomeningeal vessels. It usually manifests in the eighth decade of life, and its clinical features include lobar intracerebral hemorrhage (ICHs), progressive cognitive impairment, transient neurological episodes (TNEs) and epilepsy.6,10-12
Inflammatory CAA is a distinct subset of CAA with specific clinical and neuroradiological features characterized by an inflammatory reaction to the cerebral vascular deposits of amyloid β.7,13 The inflammatory response may be nondestructive and restricted to the lymphocytic perivascular cuffing (CAAri) or may also involve the vessel wall with transmural or intramural, and often granulomatous, inflammatory infiltrates (ABRA). Although pathologically ABRA resembles primary angiitis of the CNS, imaging findings and the colocalization of Aβ deposits, with areas of vasculitic involvement, indicate its relationship with CAAri.5,14
Based on a comprehensive review of ABRA cases by Danve et al., the mean age at the time of diagnosis is 65.2 years old and there is no gender predominance (men 53%).15 Common clinical manifestations include cognitive and behavioral changes (71%), followed by focal neurologic deficits (51%), headaches (35%) and seizures (30%).15
A comparison of ABRA and CAAri cases in a large Chinese population showed no differences in the demographic or clinical characteristics of the two groups.8 On the other hand, patients with ABRA are usually older than patients with primary CNS vasculitis (mean age 45 years) and may have a more acute disease onset.16
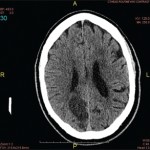
Regarding imaging findings, brain T2-weighted MRI sequences, susceptibility-weighted imaging (SWI) and fluid-attenuated inversion recovery (FLAIR) sequences have been widely used to identify patients with suspected inflammatory CAA.17,18 Typical findings include T2/FLAIR hyperintensities in the cortical white matter, usually asymmetric, with or without leptomeningeal involvement, and multiple microhemorrhages.17-20 Susceptibility-weighted imaging sequences and gradient echo (GRE) are abnormal in most patients, revealing cerebral microbleeds and superficial siderosis.6,18 The presence of leptomeningeal involvement, with or without underlying white matter abnormalities, and the absence of lobar hemorrhages help differentiate patients with inflammatory CAA from those with non-inflammatory CAA.14,21
The two pathologic subtypes of inflammatory CAA share many similar imaging findings, but ABRA cases show a higher contrast enhancement (66.7%) compared with CAAri (31.3%), possibly indicating more widespread involvement of vessel walls in ABRA.1 In a small fraction of cases (18%), inflammatory CAA can present as an infiltrative white matter lesion mimicking malignant tumors, as with our patient.5,13,14